The joint research team of UNIST including Prof. Hyun-Kon Song, Prof. Guntae Kim, and Prof. Sang Kyu Kwak (School of Energy and Chemical Engineering) has been introduced on the current issue of Angewandte Chemie International Edition. This study was about the catalyst of batteries that uses Oxygen (O2) in the air. This is the first observation showing that the intensive and intrinsic properties of electrochemical reactions possibly depend on electric conduction that have been easily thought to affect extensive properties such as the effective amount of eletroactive sites.
Hydrogen fuel cell and metal air batteries cause the electrochemical reaction when their positive poles accept O2 in the air. Those batteries use Hydrogen (H) or metals as their fuel sources and they produce the electricity by letting these materials react to O2. This Oxygen gets divided into oxygen atoms (O) in the positive pole and let them react to the electrons from the negative pole. The catalyst goes inside to accelerate this reaction.
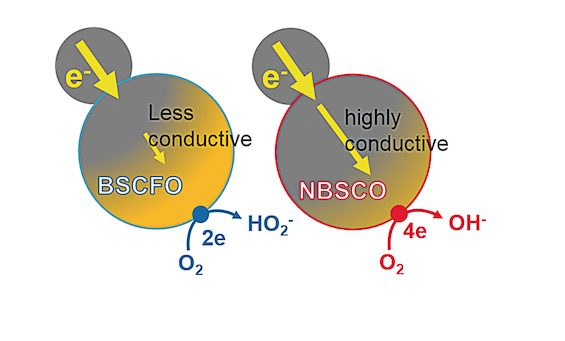
The electrochemical reaction becomes active if the conductivity of materials is high enough. Before this study was published, the normal idea was that the quantity of reaction gets increased because of the high conductivity. However, the joint team discovered that the conductivity of catalyst is very important not only on the quantity side but also on the quality side.
Prof. Song says, “Oxygen Reduction Reaction (ORR) is the process that oxygen atoms achieve electrons and it is the fundamental principle of activating metal air batteries and fuel cell. If we increase the conductivity around the catalyst, oxygen atoms get more electrons and ORR becomes more active which produces more electricity consequently.”
The research team did experiment on producing three types of oxide catalyst which has different levels of conductivity. And, the reaction of complete reduction (4 electrons reduction of Oxygen) got increased with the high conductivity. There are two different types of ORR: a more complete 4 electrons reduction of Oxygen at once and the reduction of 2 electrons at twice.
Generally, the catalysts with more complete reduction are considered as good catalysts. Therefore, electric conductivities of electrocatalysts should be more seriously considered to obtain higher energy densities in electrocatalysis-based energy devices such as fuel cells and metal air batteries.
Prof. Song says, “I could not have this research outcome without the use of Prof. Kim’s conducting oxide and Prof. Kwak’s calculation of the theory.” The lead author of this study, Dong-Gyu Lee (Researcher, School of Energy and Chemical Engineering) says, “We could define the physicochemical relationship between the catalyst and ORR. We will keep processing the project of increasing the efficiency of next generation’s fuel cells and metal air batteries’ catalyst.”
This research was sponsored by the Ministry of Science, ICT and Future Planning of Korea.
Journal Reference: Conductivity-Dependent Completion of Oxygen Reduction on Oxide Catalysts