A recent study, affiliated with UNIST has unveiled a new way to turn discarded eggshells into hydrogen, an innovative and alternative energy for the future. The new method, which is used as a catalyst for the conversion of alchohols to hydrogen, is used for the synthesis of graphene on the shell after the reaction. This technique of producing value‐added graphene and pure hydrogen, while recycling food waste is a way to “kill three birds with one stone”.
This work, led by Professor Jong‐Beom Baek and his research team in the School of Energy and Chemical Engineering at UNIST, introduces the fundamental mechanism of the selective catalytic reaction and also presents a new environmentally friendly approach for the energy‐efficient conversion of alcohols into valuable products (graphene and H2).
In this study, the team produced calcium oxide (CaO) with calcium carbonate (CaCO3), the main ingredient of an eggshell, and discovered that this can be used as a catalyst for the production of hydrogen and graphene. The use of CaO as a catalyst allowed the reaction to proceed at a lower temperature than that of conventional catalysts and produced hydrogen that could be used without any separation process. In the course of the reaction, a thin layer of carbon (C) is deposited on the calcium oxide to form the graphene, which can be easily peeled off by simple handling.
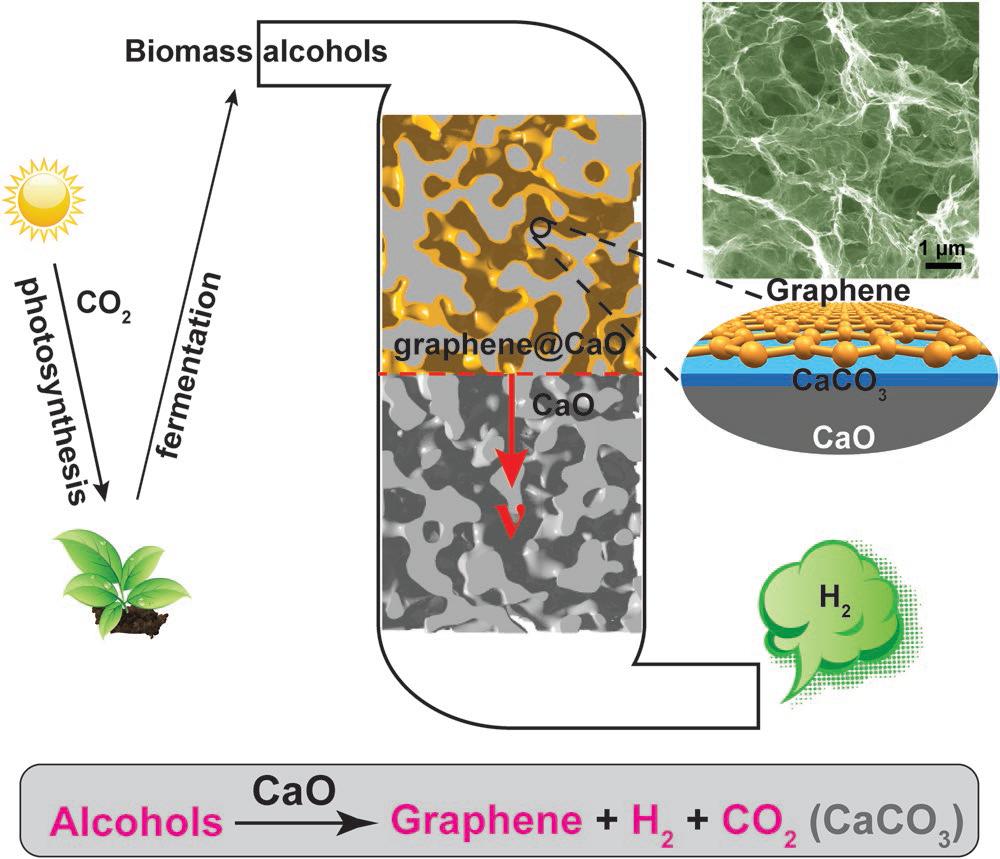
Hydrogen can be obtained from materials, containing hydrogen, which include H2O, CnH2n+2, and CnH2n+1OH. At this time, a chemical reaction is required, and a suitable catalyst is required for each reaction. And CaO showed excellent catalytic performance in ‘the process of producing hydrogen from alcohol’.
Alcohol is obtained by fermenting plants and microorganisms, so it is an eco-friendly energy source that can continue to be mass-produced in the future. Since the alcohol component is hydrogen, carbon, oxygen, it can be converted to other useful forms. Already a high temperature of over 700°C has been used to convert steam into hydrogen and carbon-based materials. However, in addition to hydrogen, by-products such as methane, carbon monoxide, and ethylene are generated at a high temperature of 700°C or higher. Therefore, it is necessary to select only the hydrogen from the produced gas, and the hydrogen production unit price is also increased.

Shown above are the discarded eggshells (left) and graphene (right).
Professor Baek has solved the disadvantages of hydrogen production using alcohol as a catalyst. The reaction temperature was lowered to 500°C by using calcium oxide made from eggshells. As a result, 99% of the produced gas was hydrogen, and BNPGr was produced in a ready-to-use state when the catalyst was removed with acid.
“Calcium oxide is an inexpensive material and it is environmentally friendly because it can be made from recycled egg shells,” says Professor Baek. “Both hydrogen and graphene are economical because they can be used without any separation process.”
Dr. Gao-Feng Han (School of Energy and Chemical Engineering, UNIST), who led the study as the first author, commence with this research by collecting eggshells from the cafeteria. He also heated the eggshells to produce CaO and used it as a catalyst for the steam reforming reaction of alcohol. In addition, he also resolved the principle, in which alcohol is converted to hydrogen and graphene, as well as the role for CaO in this process.
“The steam reforming method of alcohol using calcium oxide will have the same effect on the commercial environment where the reaction proceeds on a much larger scale,” says Dr. Han. “We hope that the results of this study will help us to recycle bioresources, including ethanol.”
This study has been jointly participated by Professor Qing Jiang and Zhi-Wen Chen from Jilin University in Changchun, China. The findings of this research have been published in the April 2019 issue of Advanced Materials.
Journal Reference
Gao‐Feng Han, et al., “Low‐Temperature Conversion of Alcohols into Bulky Nanoporous Graphene and Pure Hydrogen with Robust Selectivity on CaO,” Advanced Materials, (2019).












