A recent study, affiliated with UNIST has presented noble catalysts for water electrolysis, capable of generating hydrogen and oxygen at the same time. According to the research team, among the catalysts reported so far, these are most stable, easy to make, affordable and have excellent performance.
In this study, Professor Hyeong-seong Park, jointly with Professor Gun Tae Kim, and Professor Sang-kyu Kwak in the School of Energy and Chemical Engineering at UNIST introduced a heterostructure comprising perovskite oxides (La0.5Sr0.5CoO3–δ , LSC) and molybdenum diselenide (MoSe2) as an electrochemical catalyst for overall water electrolysis. The new catalysts are simple to synthesize and can be mass-produced, according to the research team.
‘Water Electrolysis’ technologies are regarded as the most eco-friendly and efficient way for sustainable hydrogen generation. This is a well-established technology that has been used for the decomposition of water into oxygen and hydrogen due to the passage of an electric current. At this time, a catalyst to assist the water decomposition reaction is needed. Previous studies have reported that noble metal-based catalysts, such as platinum (Pt) or iridium (Ir) show excellent catalytic performance. However, the commercialization of noble metal-based catalysts is difficult due to high-cost and low stability.
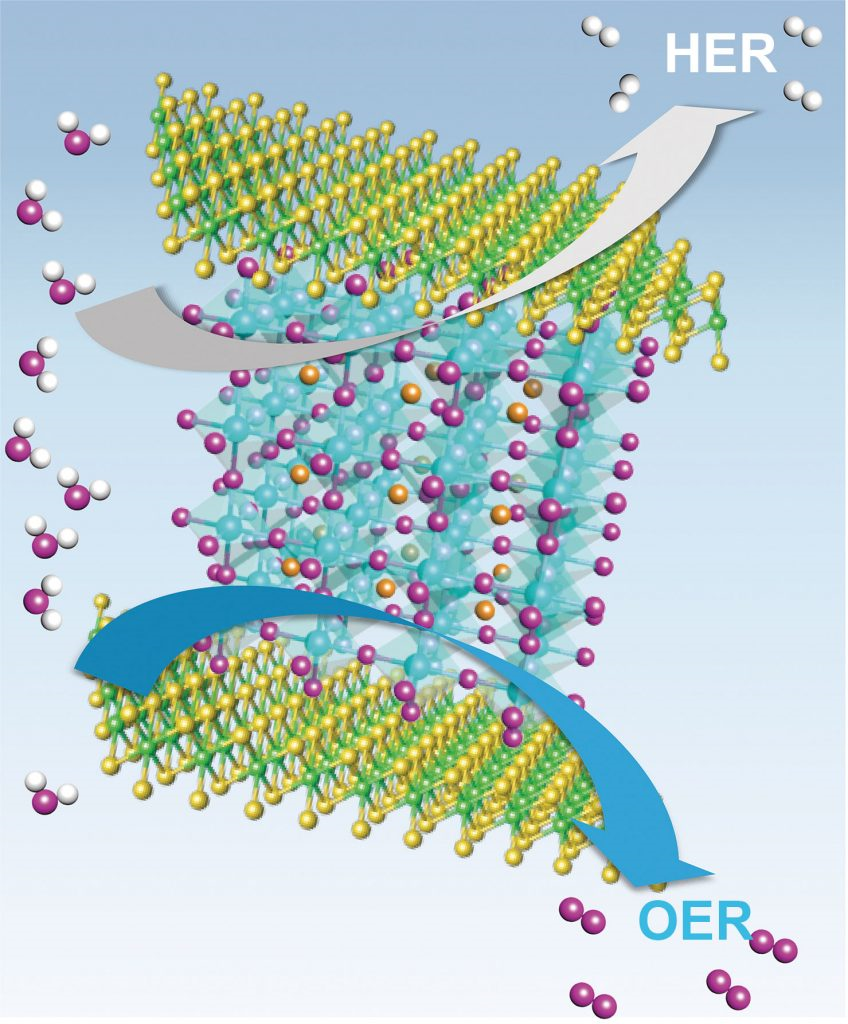
Schematic diagram of proposed charge transfer processes between MoSe2 and LSC.
In this study, the research team reports on a simple method (Ball milling technique) for the synthesis of a heterostructure catalyst where perovskite oxides (LSC) and molybdenum diselenide (MoSe2) are placed in a container, then rolled with steel metals. The new catalyst shows performance close to that of noble metal-based catalysts for both hydrogen and oxygen generation. What sets it aparts from other noble metal-based catalysts is that the new catalyst exhibits excellent catalytic performance on both sides.
In particular, the proposed catalyst exhibited excellent overall water electrolysis stability over 1000 h at a high current density of 100 mA cm–2. Previously reported catalysts suffer from electrode damage, even at the current density of 50 mA.
“Transition Metal Dichalocogenides (TMDs) are said to exhibit excellent stability, thus there had been some studies, using TMDs as catalysts for water electrolysis. However, it has been difficult to change the miconducting properties of TMDs into the properties of metals where electric current can flow freely,” says Nam Khen Oh in the doctoral program of Energy and Chemical Engineering at UNIST, the first author of the study. “In this study, some of the TMDs were converted into metal properties during the synthesis of the two materials, which greatly improved the performance and stability of the catalyst.”
The unique semiconductor–metal structural phase transitionion in the heterostructure of LSC and MoSe2 has been first discovered in this work, thus have been identified experimentally and theoretically. As electrons move from LSC to MoSe2, some structures of TMDs change, then the semiconducting property is changed to the metal property.
“The phase transition phenomenon partially appearing as electrons move between the transition metal chalcogenide and the perovskite oxide will present a new perspective on the transition metal chalcogenide phase transition,” says Professor Park. “We expect that the proposed catalyst design can be combined with various compounds, so that the potential is unlimited.”
Their findings will provide a fresh insight into the research on the electrolytic solution catalyst, which has been focused on metal-based catalysts. “Recently, most of the alkaline hydrothermal techniques are focused on the development of metal-based hydrogen production reaction catalysts,” says Changmin Kim in the Combined M.S/Ph.D. of Energy and Chemical Engineering at UNIST, the first co-author of this study. “As the catalyst for oxygen generation reaction, which has caught the backbone of the water decomposition reaction, has come out with a new catalyst showing high performance, related technology will further develop.”
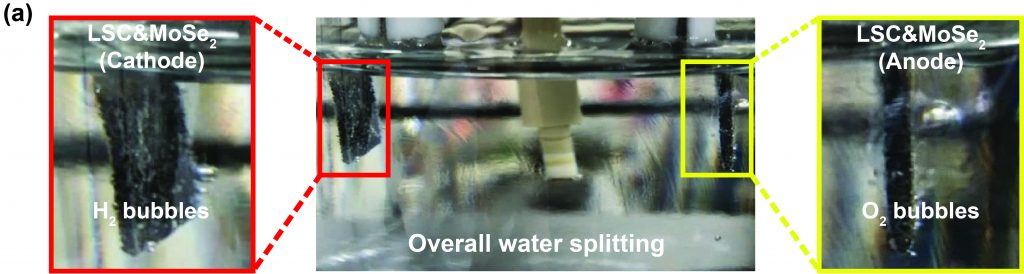
“Commercializing the hydrotreating catalysts requires simple synthesis, bulking, reproducibility, low cost, high performance, and high stability,” says Professor Kim. “Our new catalysts are expected to meet those requirements.”
This work has been supported by Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education and the Research Project Funded by U-K Brand of UNIST. It has been also supported by the Mid-Career Researcher Program through the National Research Foundation of Korea, funded by the Ministry of Science and ICT (MSIT).
Journal Reference
Nam Khen Oh, et al., “In-situ local phase-transitioned MoSe2 in La0.5Sr0.5CoO3-δ heterostructure and stable overall water electrolysis over 1000 hours,” Nature Communications, (2019).












Pingback: Ever newer shortcuts to cheaper hydrogen - Revolution-Green()