A research team, led by Professor Sung Ho Park in the Department of Biological Sciences at UNIST announced the results of a study on osteoblasts that damage joint bones in patients with rheumatoid arthritis.
In this study, the research team studied the possibility of a treatment method targeting mechanisms related to the differentiation process of osteoblasts that melt bones through enzyme reactions. First, it was confirmed that a superinhancer is formed near the NFATC1 gene, which is known to be an important factor in the formation of osteoblasts, and it is formed only in osteoblasts.
In addition, it was confirmed that an enhancer RNA, a type of non-encrypted RNA, is formed in the NFATC1 superinhand during osteoblastic cell formation.
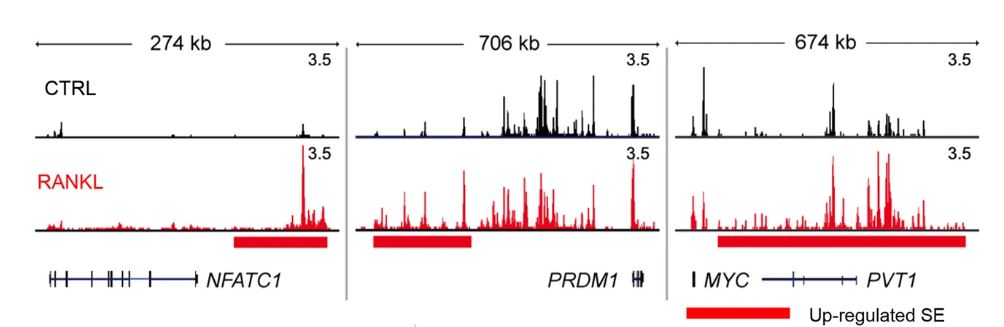
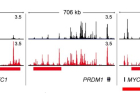
Figure 1. Identification of RANKL-sensitive super enhancers (SEs) in human osteoclasts. Human CD14+ monocytes were cultured overnight with M-CSF (20 ng ml−1) and then treated with M-CSF (CTRL) or M-CSF with RANKL (40 ng ml−1, RANKL) for three days. Shown above are the representative tracks of H3K27ac ChIP-seq at NFATc1, PRDM1, and MYC loci under the indicated conditions. Red and black bars indicate upregulated and downregulated SEs, respectively.
Non-encrypted RNA does not encode proteins, but plays an important role in regulating gene expression. In particular, due to the specificity of the molecular sequence, it can be easily targeted for treatment. In fact, we observed that interfering with NFATC1 superinhancer RNA inhibits the formation of osteoblasts together.
Through this study, it has been confirmed that NFATC1 super-in-hander RNA, which is formed during osteoblast differentiation, can be used as a treatment target.
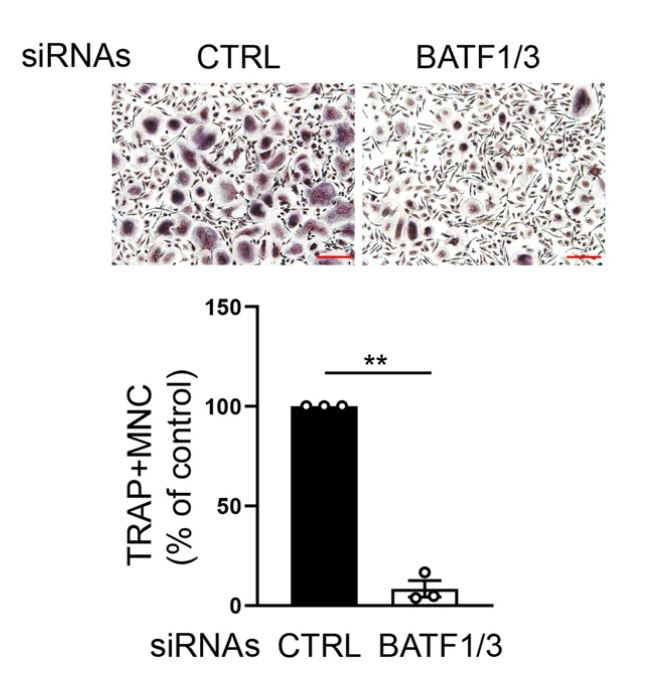
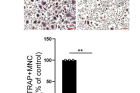
Figure 2. RANKL-induced SE-eRNAs are enriched in synovial CD14+ cells from RA patients. Shown above is the immunoblot analysis of NFATc1 expression in human CD14+ cells transfected with control or SE-eRNA:NFATc1-specific siRNA and treated for 24 h with RANKL. α-Tubulin was the loading control.
“[O]ur study is the first to identify SEs and SE-eRNAs in human osteoclasts and provides a better understanding of human osteoclast biology, thereby opening new therapeutic avenues for human pathological bone destruction,” noted the research team.
The findings of this research were made available in December 2022, ahead of its publication in the journal, Cellular and Molecular Immunology. This study has been supported by the National Research Foundation of Korea (NRF) grants, funded by the Ministry of Science and ICT (MSIT).
Journal Reference
Seyeon Bae, Kibyeong Kim, Keunsoo Kang, et al., “RANKL-responsive epigenetic mechanism reprograms macrophages into bone-resorbing osteoclasts,” Cell. Mol. Immunol. (2022).