In a groundbreaking study, Professor Won-Jin Kwak in the School of Energy and Chemical Engineering at UNIST, in collaboration with researchers from Hanyang University, have pioneered a method to significantly enhance the performance and lifespan of organic electrode-based batteries. The findings promise to accelerate the commercialization of eco-friendly batteries and pave the way for further advancements in the field.
Organic electrodes have long been recognized for their cost-effectiveness and natural abundance, making them a promising alternative to traditional lithium-ion battery materials. However, the dissolution of these active materials in the electrolyte has posed a significant challenge, limiting their practical application in batteries. To address this issue, the research team introduced diluted electrolytes as non-solvating electrolytes, effectively mitigating the physical limitations of high-concentration electrolytes (HCEs) and suppressing the dissolution of organic electrodes.
The study focused on perylene-3,4,9,10-tetracarboxylic dianhydride (PTCDA), a prominent organic electrode material, and demonstrated remarkable improvements in capacity retention and rate performance when using diluted electrolytes. Over 1000 cycles, the PTCDA-based battery achieved an impressive 91% capacity retention at 1000 mA g−1. Through a combination of electrochemical and spectroscopic measurements, as well as molecular dynamics simulations, the team successfully inhibited the dissolution of the active material and mitigated the detrimental shuttle effect, thereby preventing capacity loss.
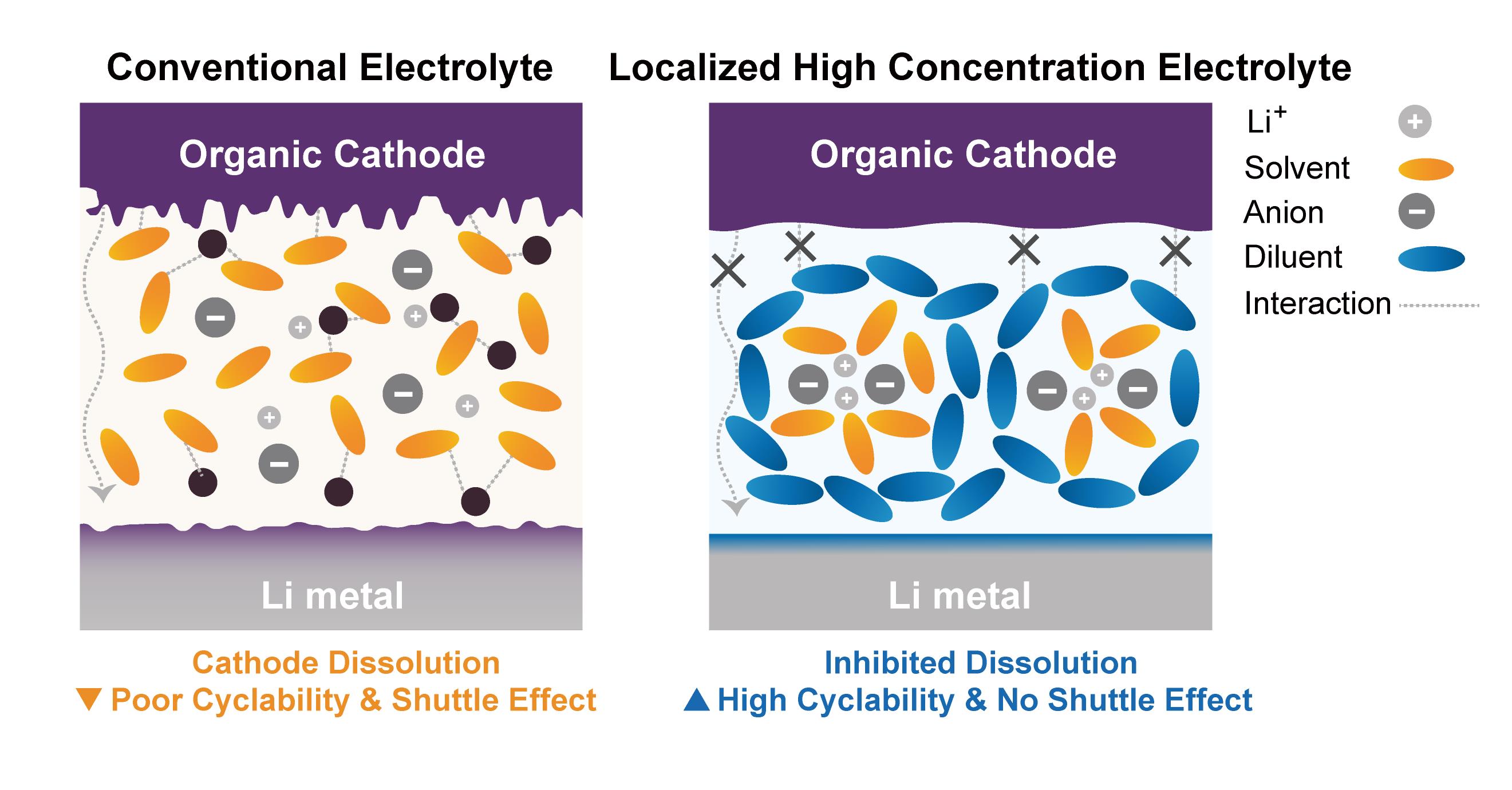
Figure 1. Schematic illustration of organic electrode-based LIB using different types of electrolytes. The strong interaction energy between ethylene glycol dimethyl ether (DME) and the organic electrode induces the dissolution of the organic electrode, compromising the stability of the electrode. Conversely, TTE in the LHCE reduces the interaction energy between the electrolyte and organic electrode, thereby suppressing dissolution.
This research presents a promising strategy for achieving highly reversible organic electrode-based lithium-ion batteries, opening up new possibilities for the development of more efficient and sustainable energy storage solutions. By utilizing non-solvating electrolytes, the team has overcome the limitations associated with organic electrode materials and demonstrated a path towards extending the lifespan of these eco-friendly batteries.
Professor Kwak emphasized the significance of this breakthrough by saying “The development of non-solvating electrolytes provides an effective approach to suppress the elution of organic electrode materials, without compromising capacity or output. This study represents a major step towards practical applications of organic electrode-based batteries.”
The research team further validated their findings through computational methods and experimental verification, confirming that the use of the developed electrolyte led to the effective suppression of elution. In more than 1,000 charge/discharge experiments, over 80% of the battery capacity was retained, in sharp contrast to conventional electrolytes, which exhibited less than 50% capacity after just 20 charge/discharge cycles.
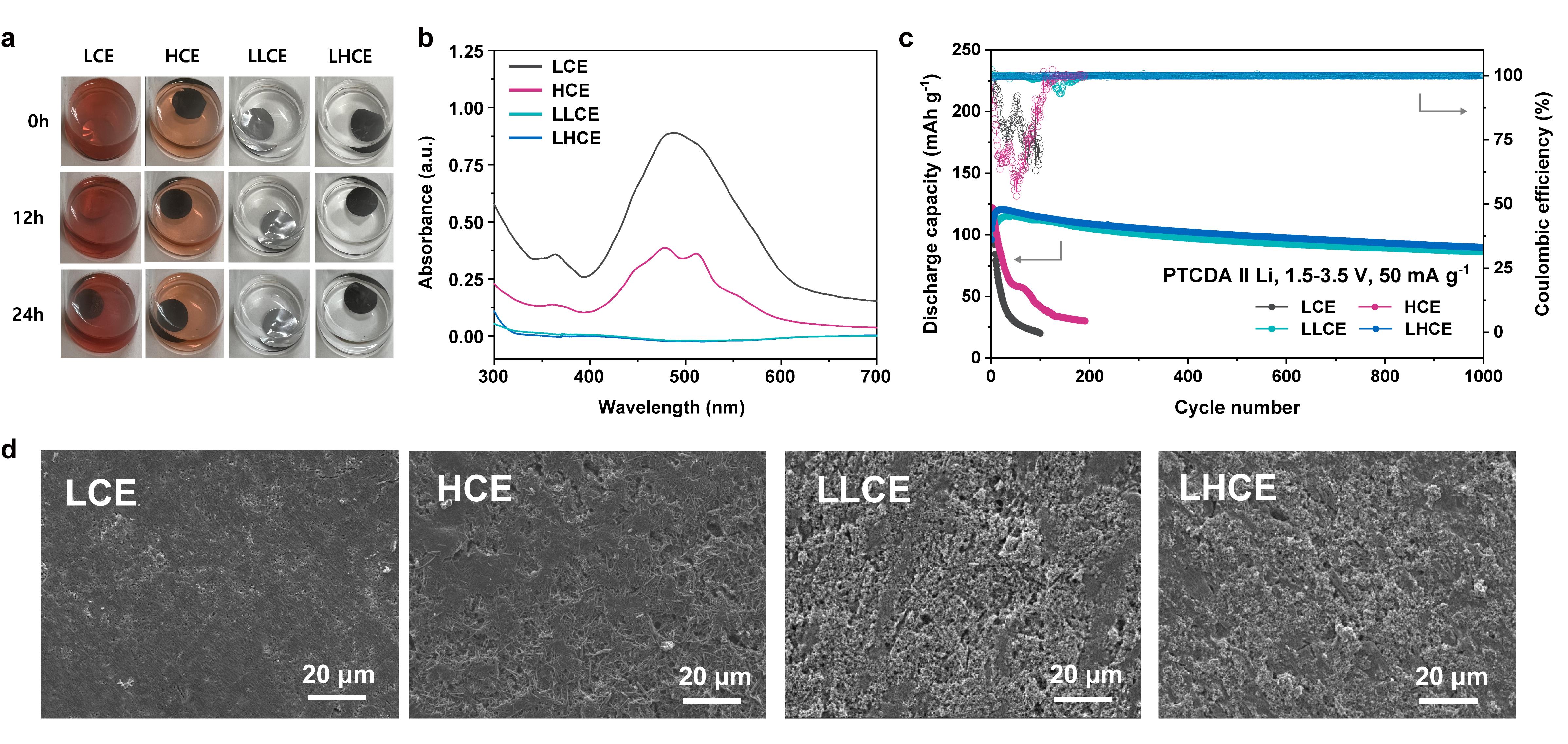
Figure 2. Effect of inhibiting the dissolution of organic electrodes in diluted electrolyte.
The potential of organic electrode-based secondary batteries using non-solvating electrolytes holds great promise in overcoming resource depletion and rising material prices. As researchers continue to tackle the challenges associated with these batteries, this study sets the stage for future breakthroughs and advancements in the field.
The findings of this research have been published in the Advanced Energy Materials on January 19, 2024. The research was supported by the National Research Foundation of Korea (NRF) and the Ministry of Science and ICT (MSIT).
Journal Reference
Hyun-Wook Lee, Youngoh Kim, Joo-Eun Kim, et al., “Diluents Effect on Inhibiting Dissolution of Organic Electrode for Highly Reversible Li-Ion Batteries,” Adv. Ener. Mater., (2024).












