A groundbreaking technology that harnesses solar energy to produce high-efficiency ammonia (NH3) has been unveiled by a research team affiliated with UNIST. Led by Professor Sung-Yeon Jang and Professor Ji-Wook Jang from the School of Energy and Chemical Engineering at UNIST, in collaboration with Professor Thomas F. Jaramillo from Stanford University, the team has developed an eco-friendly perovskite-based photoelectrode system for NH3 production that has surpassed the commercialization standard of the U.S. Department of Energy (DOE) by an impressive 1.7 times, setting a new world record in ammonia production efficiency.
The innovative system operates on the principle of reducing nitrate (NO3-) in water to produce NH3 using solar energy. This method not only offers a more environmentally friendly alternative to the conventional Haber-Bosch process, which heavily relies on fossil fuels, but also opens up opportunities for the synthesis of high-value compounds used in various industries such as fertilizers, food, and pharmaceuticals.
Key to the success of this technology is the development of a highly efficient photoelectrode system that combines perovskite solar cells with a ruthenium (Ru) catalyst on titanate nanosheets (TiNS). By protecting the perovskite material with Field’s metal and integrating it with the catalyst for NH3 production, the research team has achieved unparalleled performance and durability in NH3 production.
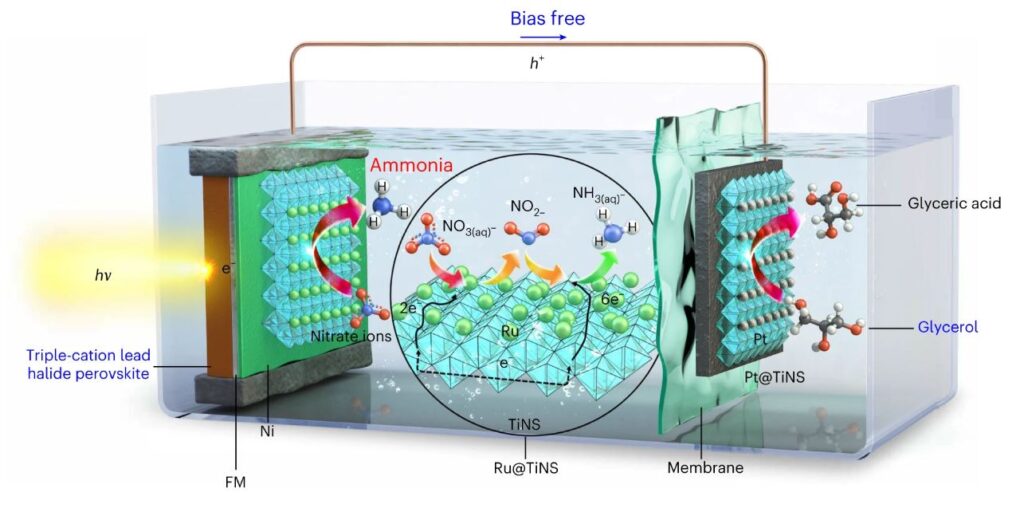
Figure 1. Schematic of the PEC cell used for NH3 production. Ru@TiNS/Ni/perovskite photocathode is combined with Pt@TiNS anode to achieve a simultaneous bias-free NH3 production and glycerol valorization.
Noteworthy is the use of glycerol as a reactant, which enables the production of NH3 without the need for external voltage. By optimizing the oxidation reaction of glycerol with the voltage generated by the photoelectrodes, the team has demonstrated a remarkable maximum ammonia production rate of 1745 μgNH3 cm-2h-1, far surpassing the commercialization standard of the U.S. Department of Energy (DOE).
In a statement, Professor Ji-Wook Jang highlighted the potential of this technology for eco-friendly fuel production, stating, “Through this study, we have demonstrated the production of NO3-, a main source of contamination in water, while at the same time oxidizing, glycerol, a low-value byproduct derived from biomass, to produce a higher-value glyceric acid (GA).” He further added, “This technology holds immense potential for the production of eco-friendly fuels.”
Professor Sung-Yeon Jang emphasized the significance of advancing solar fuel production, stating, “Our research represents a significant advancement in solar fuel production, surpassing commercialization standards and paving the way for a more sustainable future in ammonia production.”
The research findings have been published in the latest issue of Nature Catalysis, a prestigious journal in catalytic research, and have been co-authored by Ahmad Tayyebi, Rashmi Mehrotra, Muhibullah Al Mubarok, and Jiun Kim from the Combined M.S./Ph.D. program of Energy and Chemical Engineering at UNIST. This groundbreaking research was made possible through support from the Ministry of Science and ICT (MSIT), the National Research Foundation (NRF) of Korea, and the Basic Research Laboratory (BRL) Support Project.
Journal Reference
Ahmad Tayyebi, Rashmi Mehrotra, Muhibullah Al Mubarok, et al., “Bias-free solar NH3 production by perovskite-based photocathode coupled to valorization of glycerol,” Nat. Catal., (2024).