A research team, affiliated with UNIST has announced the development of an innovative nonlinear imaging technique capable of visualizing internal biological tissues in 3D using ordinary light sources, such as laser pointers, instead of costly ultrafast pulsed lasers costing hundreds of millions of Korean won.
Led by Professor Jung-Hoon Park and Professor Jinmyoung Joo in the Department of Biomedical Engineering at UNIST, the research team has utilized specialized nanoparticles to create a nonlinear fluorescence microscopy technology that operates solely with continuous-wave (CW) lasers. This breakthrough achieves resolution and tissue penetration depths comparable to those of conventional systems that rely on expensive femtosecond pulsed lasers, and it can also be applied for precise photodynamic therapy (PDT) that selectively targets lesions without damaging surrounding tissues.
Biological tissues scatter light extensively, making clear internal imaging difficult. To overcome this, techniques such as multiphoton microscopy focus on inducing fluorescence only near the focal point, filtering out background noise caused by scattering. However, multiphoton microscopy requires femtosecond pulsed lasers to deliver high photon densities necessary for simultaneous multi-photon absorption, limiting accessibility in clinical and laboratory settings due to high costs.
The research team circumvented this limitation by employing upconverting nanoparticles (UCNPs). When injected into biological tissues via blood flow, these nanocrystals absorb photons from a simple CW laser and emit light at ultraviolet (UV), blue, or near-infrared (NIR) wavelengths. Due to their nonlinear optical properties—where emission intensity depends on the square or cube of the excitation light’s intensity—significant fluorescence occurs only in regions where the light is highly concentrated, such as the focal point.
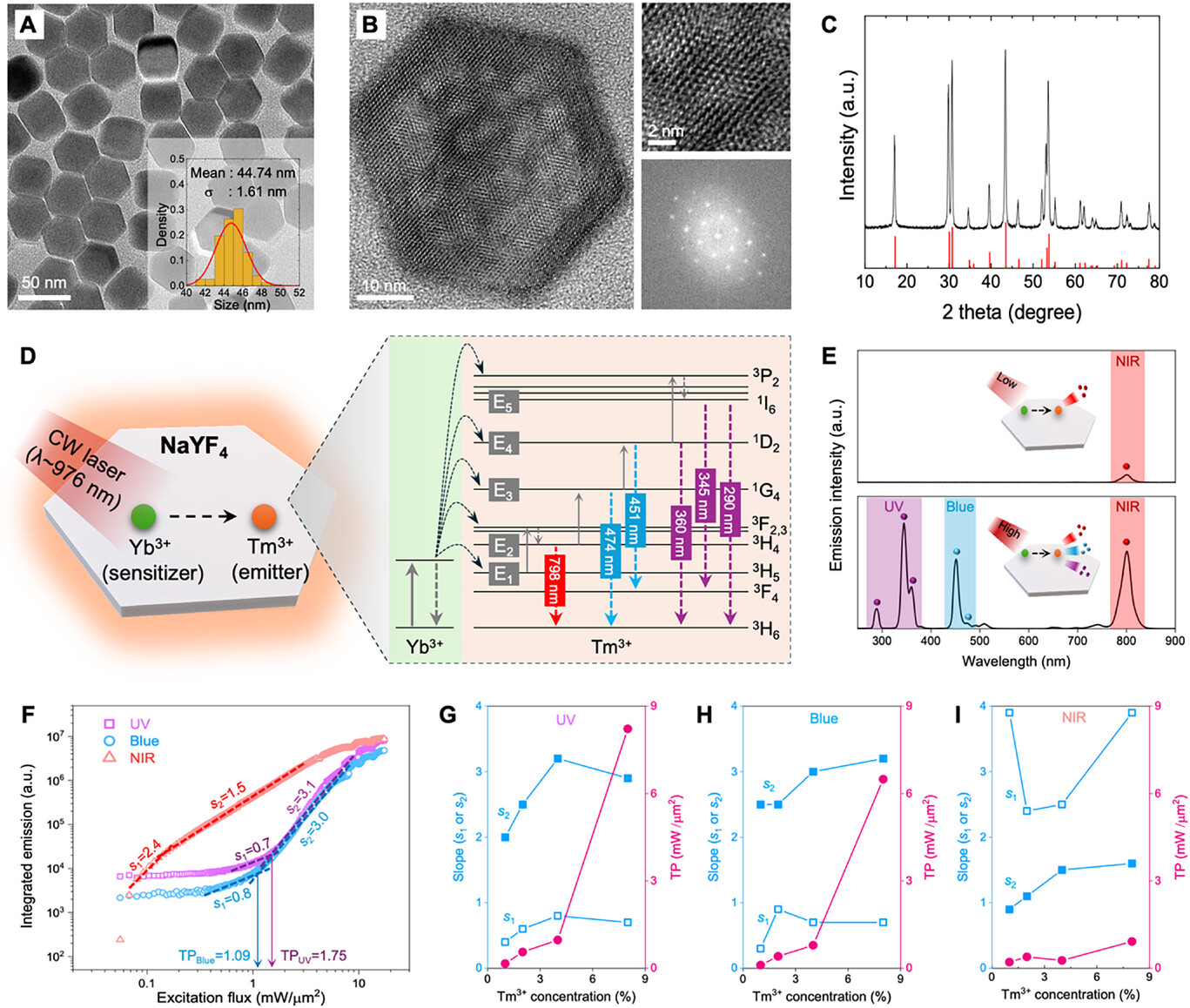
Figure 1. Schematic image, illustrating the nonlinear optical response of NaYF4 crystals co-doped with Yb3+ and Tm3+ ions.
Using this approach, the team successfully captured high-resolution, 3D images of cerebral blood vessels in living mice at depths of approximately 800 micrometers—about six times deeper than traditional confocal microscopy and comparable to multiphoton microscopy. Furthermore, the system enabled real-time visualization of blood flow at 30 frames per second across a broad field of view, illustrating its potential for rapid, in vivo vascular studies.
Beyond imaging, this technology allows for depth-selective, three-dimensional photomodulation within turbid media. By harnessing the localized fluorescence generated only at the focus, clinicians can activate photosensitive agents precisely at target sites, minimizing collateral damage to surrounding tissues. The team demonstrated this capability by activating UV-responsive materials selectively within tissue depths using UCNP-generated UV light.
This advancement offers a cost-effective alternative to traditional high-end microscopy tools, providing high-resolution deep tissue imaging and precise phototherapy without the need for expensive femtosecond lasers. When combined with existing diagnostic equipment such as MRI, this technology could significantly enhance the ability to monitor cerebral blood flow, metabolic responses, and other physiological processes in clinical settings.
The research was supported by the Ministry of Science and ICT (MSIT), the National Research Foundation of Korea (NRF), the Institute for Information & Communications Technology Planning & Evaluation (IITP), the Korea Dementia Research Center (KDRC), the Korean Fund for Regenerative Medicine (KFRM), and the TJ Park Foundation. The findings were published on May 12 in Advanced Materials, a leading journal in materials science, where the paper was featured on the cover story for that issue.
The research was supported by the Ministry of Science and ICT (MSIT), the National Research Foundation of Korea (NRF), the Institute for Information & Communications Technology Planning & Evaluation (IITP), the Korea Dementia Research Center (KDRC), the Korean Fund for Regenerative Medicine (KFRM), and the TJ Park Foundation. The findings were published on May 12 in Advanced Materials, a leading journal in materials science, with the paper featured on the cover of that issue.
Journal Reference
Jeongmo Kim, Seunghun Lee, Yundon Jeong, et al., “Unlocking Multimodal Nonlinear Microscopy for Deep-Tissue Imaging under Continuous-Wave Excitation with Tunable Upconverting Nanoparticles,” Adv. Mater., (2025).













