A research team, affiliated with UNIST has unveiled a groundbreaking technology that transforms nitrates found in wastewater into ammonia, a vital chemical and promising energy carrier, without carbon emissions. This advancement not only offers a sustainable method for ammonia production but also contributes to wastewater purification efforts.
Jointly led by Professors Kwanyong Seo and Ji-Wook Jang from the School of Energy Chemistry at UNIST, the research team announced that they have successfully engineered a solar-powered photoelectrochemical (PEC) system capable of converting nitrate pollutants into ammonia under ambient conditions.
Ammonia is an essential chemical used globally in agriculture and industry, with an annual consumption exceeding 150 million tons. Its high hydrogen content also positions it as a promising candidate for next-generation energy storage and transportation solutions. However, the prevailing industrial method— the Haber-Bosch process— relies heavily on high-temperature and high-pressure conditions, resulting in significant greenhouse gas emissions.
Their innovative PEC system utilizes sunlight to drive nitrate reduction without the need for external electrical power, leveraging wastewater as a raw material. Nitrates, when present at high concentrations, pose health risks such as methemoglobinemia and gastric cancers. The new technology selectively reduces nitrates to ammonia, effectively addressing both water pollution and sustainable ammonia synthesis.
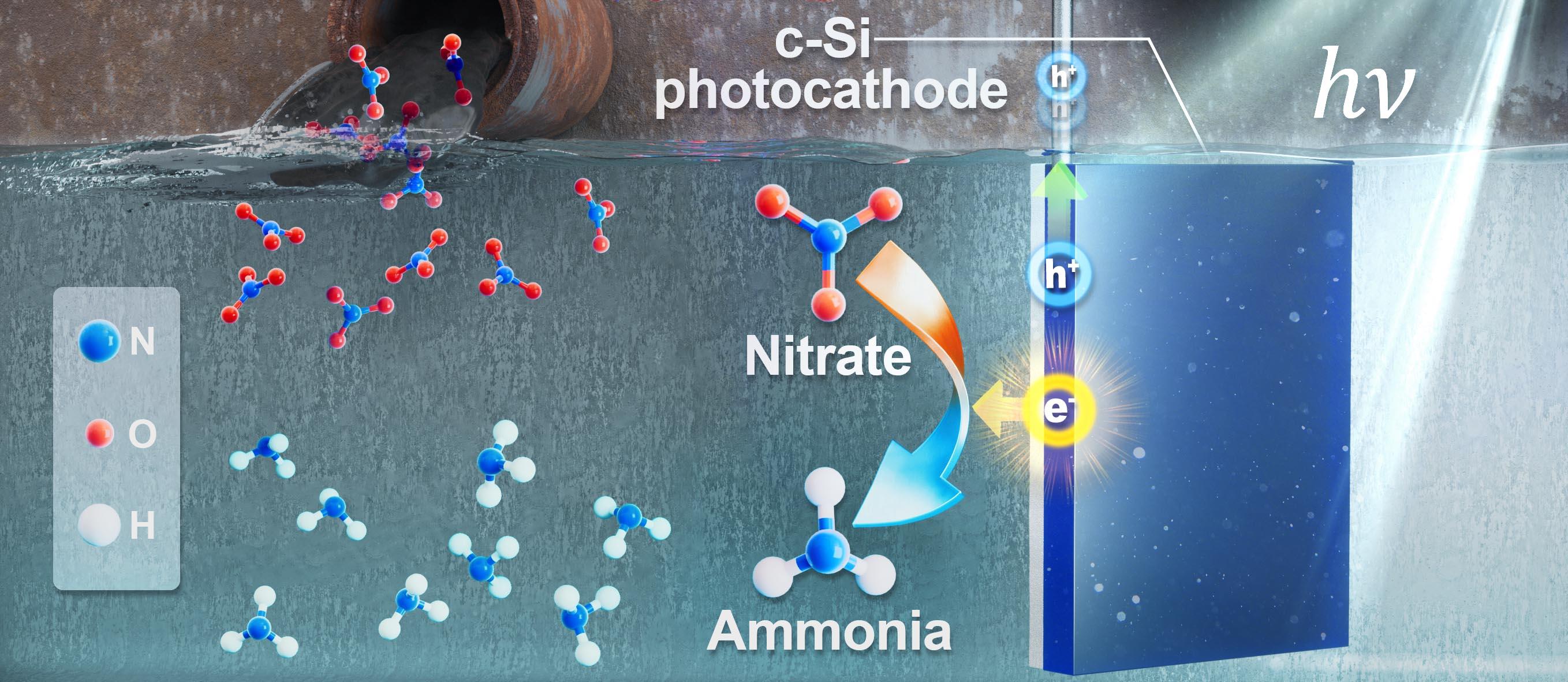
A schematic illustration of the photoelectrochemical nitrate reduction reaction (PEC NO3RR).
The system comprises a silicon-based photocathode paired with a nickel foil catalyst. Sunlight excites the silicon, generating electrons that are then channeled via the nickel catalyst to reduce nitrates to ammonia. A key innovation lies in the formation of a thin layer of nickel hydroxide (Ni(OH)₂) on the catalyst surface during operation, which suppresses competing reactions like hydrogen evolution and enhances selectivity toward ammonia production.
This mechanism was confirmed through extensive experiments and quantum mechanical calculations. Professor Suthsu Ryu from UNIST’s Department of Physics, utilizing density functional theory (DFT) simulations, demonstrated that Ni(OH)₂ offers active sites that favor nitrate reduction to ammonia, lowering the energy barriers involved.
Remarkably, the system achieved a record-high ammonia production rate of 554 micrograms per square centimeter per hour without any external bias, outperforming previous technologies by more than 50%. The system maintained its performance on a larger 25 cm² scale, indicating promising potential for real-world application.
Professor Seo emphasized, “Converting nitrate pollutants into ammonia not only purifies water but also contributes to carbon neutrality. Our goal is to develop large-scale, practical PEC devices capable of producing ammonia outdoors, directly utilizing sunlight and wastewater as resources.”
This research was supported by the National Research Foundation of Korea (NRF), the Korea Institute of Energy Technology Evaluation and Planning (KETEP), as well as the UNIST Central Research Facilities (UCRF) for the support of its facilities and equipment. The findings of this research were published online in Advanced Materials on June 22, 2025.
Journal Reference
Wonjoo Jin, Hyunju Go, Juyeon Jeong, “Nickel Hydroxide Catalyzed Bias-free Photoelectrochemical NH3 Production via Nitrate Reduction,” Adv., Mat., (2025).