A research team, affiliated with UNIST has unveiled a novel technology that enables hydrogen to be stored within polystyrene-derived materials, particularly those originating from Styrofoam. This advancement not only offers a solution to the low recycling rate of polystyrene—less than 1%—but also makes hydrogen storage and transportation more practical and accessible, addressing the challenges associated with handling gaseous hydrogen.
Led by Professor Kwangjin An from the School of Energy and Chemical Engineering at UNIST, in collaboration with Dr. Hyuntae Sohn from KIST and Professor Jeehoon Han from POSTECH, the team successfully designed a comprehensive, closed-loop system to convert waste polystyrene into a liquid organic hydrogen carrier (LOHC). This innovative process enables efficient hydrogen storage, retrieval, and reuse.
LOHC molecules store hydrogen within their cyclic chemical structure, allowing safe, stable storage at room temperature and pressure. Their liquid form facilitates long-term storage and is compatible with existing oil transportation infrastructure—making this approach particularly promising for the hydrogen economy.
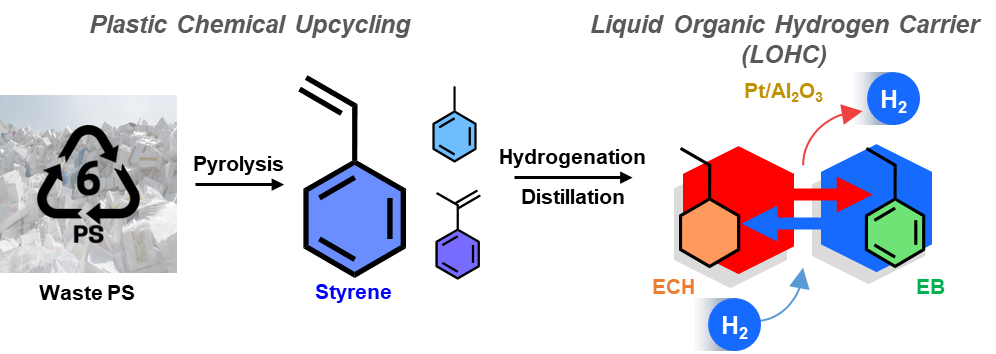
Figure 1. Schematic image illustrating the overall study of the research.
The reported approach exploits the aromatic-rich structure of polystyrene. When heated, polystyrene decomposes into low-molecular-weight aromatic compounds such as styrene and toluene. These compounds react with hydrogen at elevated temperatures to store hydrogen, which can later be released via catalytic dehydrogenation.
Catalysts are central to this process. Ruthenium catalysts facilitate hydrogen absorption, while platinum catalysts enable hydrogen release. Notably, the researchers discovered that the catalytic performance of platinum-supported catalysts varies significantly depending on the support structure. Among various options, nanosheet-assembled aluminum oxide demonstrated remarkable reactivity and stability, improving hydrogen release efficiency.
To prevent catalyst deactivation due to impurity buildup, the team implemented a distillation step to selectively remove polycyclic compounds and other contaminants, thereby extending catalyst lifespan and ensuring process durability.
Additionally, the researchers optimized the process for energy efficiency and economic viability by utilizing waste heat generated during the reaction—through the combustion of residual byproducts—reducing external energy consumption and increasing the throughput of polystyrene waste conversion.
Commenting on their findings, the research team stated, “This is the first-ever demonstration of converting waste polystyrene into a practical hydrogen storage medium. Our approach tackles two significant environmental challenges—plastic waste recycling and hydrogen storage—simultaneously.” They further added, “We believe this technology has strong potential for industrial application and policy development in the future.”
This research was supported by the Ministry of Science and ICT (MSIT), the National Research Foundation of Korea (NRF), and the Engineering Research Center (ERC) of Excellence Program. The findings were published as a back cover of the August 2025 issue of ACS Catalysis.
Journal Reference
Hyeongeon Lee, Yoondo Kim, Heedo Ryou, et al., “Upcycling Post-Consumer Polystyrene Waste into Liquid Organic Hydrogen Carriers,” ACS Catal., (2025).