In an unexpected discovery, researchers have discovered that cancer cells can exploit signals typically associated with low oxygen conditions—known as hypoxia—to promote tumor invasion and metastasis, even when oxygen levels are sufficient. This groundbreaking finding, led by a research team at UNIST, illuminates a previously unrecognized cellular circuit that enables melanoma cells to mimic hypoxic signals, thereby enhancing their metastatic potential. Importantly, the team demonstrated that disrupting this circuit could effectively suppress cancer spread.
Led by Professor Byoung Heon Kang in the Department of Biomedical Engineering at UNIST, the team identified a novel mechanism in melanoma cells where the protein HIF1α, a master regulator activated under low oxygen, is stabilized in normoxic (oxygen-rich) environments through the accumulation of mitochondrial reactive oxygen species (ROS). Rather than oxygen deficiency, it is ROS that triggers HIF1α, leading to increased tumor invasiveness.
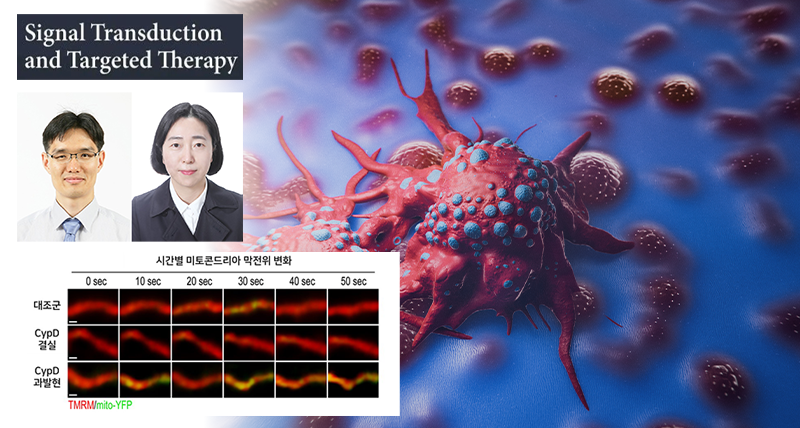
Crucially, the researchers uncovered that activating a mitochondrial protein called Cyclophilin D (CypD) can break this pseudohypoxic signaling loop. When CypD is activated, it opens a mitochondrial channel known as the permeability transition pore (mPTP), reducing ROS build-up inside the cell. This, in turn, restores the normal degradation of HIF1α, preventing the cancer cells from acquiring invasive properties.
In animal models, local delivery of a gene therapy vector to overexpress CypD in melanoma tissues significantly impeded metastasis to lymph nodes and lungs—the primary sites of melanoma spread. Notably, this intervention did not affect the size of the primary tumor, suggesting its potential as a targeted anti-metastatic therapy.
Professor Kang explains, “Melanoma’s accessibility on the skin makes it an excellent candidate for localized gene therapy. Our findings suggest that boosting CypD activity could serve as a powerful strategy to prevent metastasis, especially when combined with existing immunotherapies, potentially producing a synergistic effect.”
He adds, “Targeting this mitochondrial circuit not only hinders tumor invasion but could also modulate the tumor’s blood vessel formation and immune environment, paving the way for novel, comprehensive anti-cancer treatments.”
This study was published in the online version of Signal Transduction and Targeted Therapy (Impact Factor = 52.7), a leading journal in cancer research, on July 24, 2025. The research was supported by the National Research Foundation of Korea (NRF) and the Ministry of Science and ICT (MSIT).
Journal Reference
Hye-Kyung Park, Sung Hu, So Yeon Kim, et al., “Pseudohypoxic stabilization of HIF1α via cyclophilin D suppression promotes melanoma metastasis,” (2025).