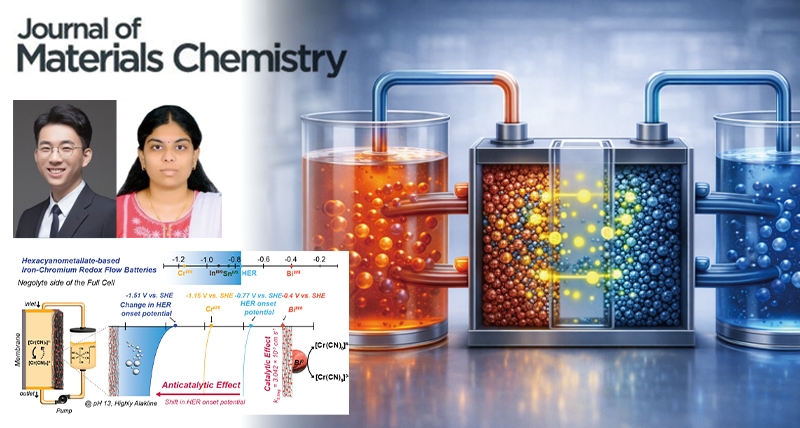
A research team, affiliated with UNIST has achieved a major breakthrough in the development of cost-effective, large-scale energy storage systems (ESS)—specifically, iron–chromium redox flow batteries (ICRFBs). Known for their safety, affordability, and suitability for grid-level applications, these batteries offer a reliable power source for high-demand facilities, like data centers, all while eliminating the risks associated with flammable electrolytes.
Led by Professor Hyun-Wook Lee of the Department of Energy Chemistry, the team demonstrated that coating the electrodes with bismuth (Bi) dramatically enhances battery performance. This simple surface modification accelerates the chromium redox reactions by over ten times and suppresses parasitic hydrogen evolution, resulting in higher energy efficiency and longer cycle life.
ICRFBs utilize aqueous solutions containing iron and chromium, stored separately and circulated through electrodes during charging and discharging. Their water-based electrolytes eliminate explosion risks, while the abundance and low cost of iron and chromium make these batteries highly attractive for large-scale deployment.
However, challenges such as sluggish chromium reaction kinetics and side reactions—particularly hydrogen evolution—have limited their practical use. Overcoming these issues is critical for achieving durable, high-efficiency operations.
In their latest publication, Professor Lee and his team introduced an innovative approach rooted in interface engineering. By electrodepositing bismuth onto the electrode surfaces, they achieved two key outcomes: (1) Accelerated charge transfer kinetics for Cr³⁺/Cr²⁺ (approximately 10× increase) and (2) Suppressed hydrogen evolution reaction (HER), shifting its onset to more negative potentials.
This dual catalytic and anticatalytic behavior enables stable operation over more than 500 cycles, with an average energy efficiency of 75.22%. Notably, the Coulombic efficiency reached 99.29%, indicating that nearly all injected electrons contributed to useful electrochemical reactions.
Professor Lee shared, “This work demonstrates that electrode interface design can do more than just catalyze desired reactions; it can also be employed to deliberately suppress undesired side reactions. This dual strategy opens new avenues for designing more durable and efficient aqueous RFBs.”
Reflecting on the broader significance, he added, “The discovery of hexacyanochromate as an anion-based chromium redox mediator was a pivotal moment in my scientific career. Since our initial report in Advanced Energy Materials (2023), our group has continued to explore this chemistry, leading to multiple publications and deeper insights into how to leverage anion-based redox reactions for practical energy storage solutions.”
Professor Lee concluded, “We are excited to continue pushing the boundaries of anion-based redox chemistry to develop longer-lasting, safer, and more economical energy storage technologies—crucial components for a sustainable energy future.”
The findings of this research have been published in the Journal of Materials Chemistry A on January 7, 2026. The study has been supported by the National Research Foundation of Korea (NRF) and the Ministry of Science and ICT (MSIT).
Journal Reference
Vithiya Muralidharan, Ryeong-ah Kim, Ji-Eun Jang, and Hyun-Wook Lee, “Electrocatalyst-induced kinetic modulation of anion-based redox mediators in aqueous iron–chromium redox flow batteries,” J. Mater. Chem. A., (2026).