A research team, affiliated with UNIST has announced that they have succeeded in developing a mechanochemical approach that can greatly increase the yield of ammonia (NH3), using iron (Fe) catalyst with potassium (K) as a promoter.
Ammonia (NH3) is a substance that synthesizes nitrogen and hydrogen by chemical reactions and is widely used in fertilizers and chemical industries and is in the spotlight as a hydrogen carrier. Ammonia has a hydrogen storage density per unit volume 1.7 times higher than that of liquefied hydrogen, so it can store a large amount of hydrogen. In addition, it is easy to transport and distribute because it is easily liquefied at natural temperatures and atmospheric pressure.
To make NH3, which consists of three hydrogen atoms and one nitrogen atom, one must first break the strong bond that holds two nitrogen atoms together. Since the early 1900s, the Haber-Bosch process has been the primary method for industrial production of NH3 from nitrogen (N2) and hydrogen (H2) under high temperature and pressure conditions. In this process, potassium oxide (K2O) is frequently used as a promotor in industrial ammonia synthesis because it can easily stabilize potassium (K) at high temperatures. However, potassium oxide contains oxygen, which hinders the bonding of nitrogen, thereby lowering the performance of iron catalysts.
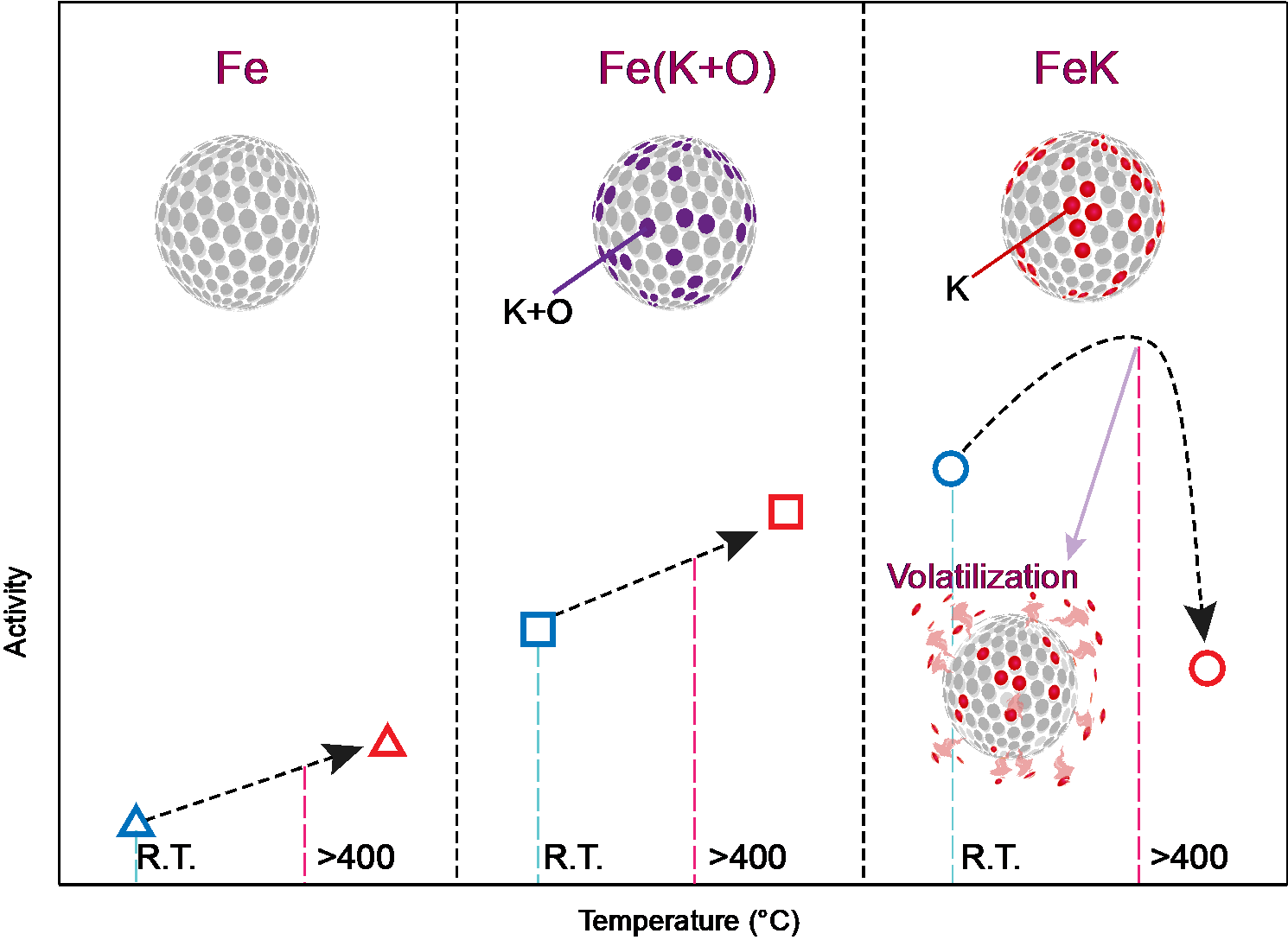 Figure 1. Above is the schematic image of Fe catalytic activity with different promotors depending on reaction temperature.
Figure 1. Above is the schematic image of Fe catalytic activity with different promotors depending on reaction temperature.
To improve this, the research team devised a method of synthesizing ammonia at low temperature and low pressure via mechanochemical process. Instead of K2O, potassium (K) was used as a direct reaction promoter to effectively break the bond of nitrogen. With this method, the team achieved catalytic performance about 30% higher than potassium oxide and ammonia yield (94.5%), about 12% higher than when using only iron catalysts (82.5%). Furthermore, the minimum rotation speed for hydrogenation was only 100 r.p.m., which was considerably lower than using pure Fe (350 r.p.m.), noted the research team.
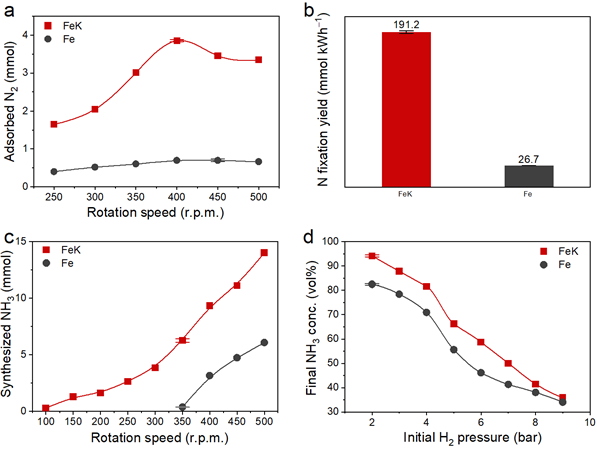
Figure 2. N2 dissociation kinetics. The amount of adsorbed N2 with respect to rotation speed. The total rotating cycles for FeK were 108,000 cycles, and for pure Fe were 240,000 cycles. Data were normalized to 30,000 cycles (1 hour at 500 r.p.m.)
“We found a new opportunity to use pure metallic K as a promotor to boost the efficiency of ammonia synthesis for commercial production without the risk of oxygen poisoning of the Fe catalyst,” noted Professor Baek.
The findings of this research have been published in the April 2023 issue of Nature Communications. This study has been supported by the Creative Research Initiative, the Young Researcher, the Basic Science Research, and the BK21 Plus programs through the National Research Foundation (NRF) of Korea and the U-K Brand and CNI Projects of UNIST, National Natural Science Foundation of China, the Program for JLU Science and Technology Innovative Research Team, and the Fundamental Research Funds for the Central Universities.
Journal Reference
Jong-Hoon Kim, Tian-Yi Dai, Mihyun Yang, et al., “Achieving volatile potassium promoted ammonia synthesis via mechanochemistry,” Nat. Commun., (2023).
















