A team of researchers from UNIST has achieved a remarkable breakthrough in the production of clean gas raw materials, particularly green hydrogen. By developing a hydrogel thin film technology, the team has paved the way for a revolution in gas-producing electrodes, significantly enhancing production efficiency and expediting the commercialization of green hydrogen production.
Under the leadership of Professor Jungki Ryu and Professor Dong Woog Lee in the School of Energy and Chemical Engineering at UNIST, the research team has successfully created a large-area hydrogel thin film technology that can be universally applied to gas-evolving electrodes. By leveraging the application of a hydrogel thin film, akin to those commonly found in cosmetics, the researchers have made remarkable strides in boosting the production efficiency of gaseous raw materials generated through electrochemical reactions. This cutting-edge technology facilitates the seamless release of gas raw materials, mitigating the accumulation on the electrode surface, which has long been a significant challenge in gas evolution reactions.
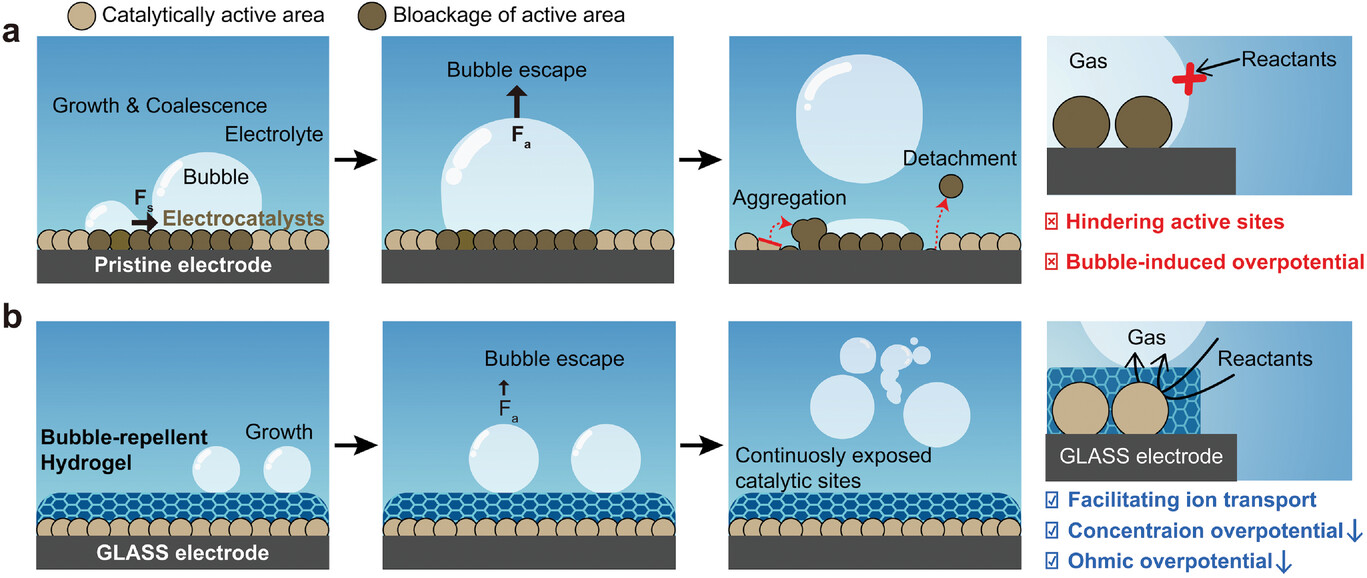
Figure 1, GLASS for efficient and stable gas evolution reactions. The schematic illustration shows a) the detrimental effect of adsorbed gas bubbles on the performance and stability of electrodes for gas evolution reactions and b) the underlying mechanism of GLASS to improve their efficiency and stability by addressing such extrinsic effects. Fa and Fs represent adhesion and shear forces, respectively.
During electrochemical reactions, such as water electrolysis, gases like hydrogen, oxygen, and nitrogen are produced. However, these gases often become trapped as air bubbles on the electrode surface, impeding electrolyte permeation and diminishing overall efficiency. To overcome this critical issue, the research team ingeniously coated the electrode surface with a hydrogel layer featuring porous structures, thereby enabling efficient gas exchange and the swift removal of gas bubbles. The hydrogel employed in this study is a readily available material with high hydrophilicity, commonly utilized in cosmetics, ointments, and diapers.
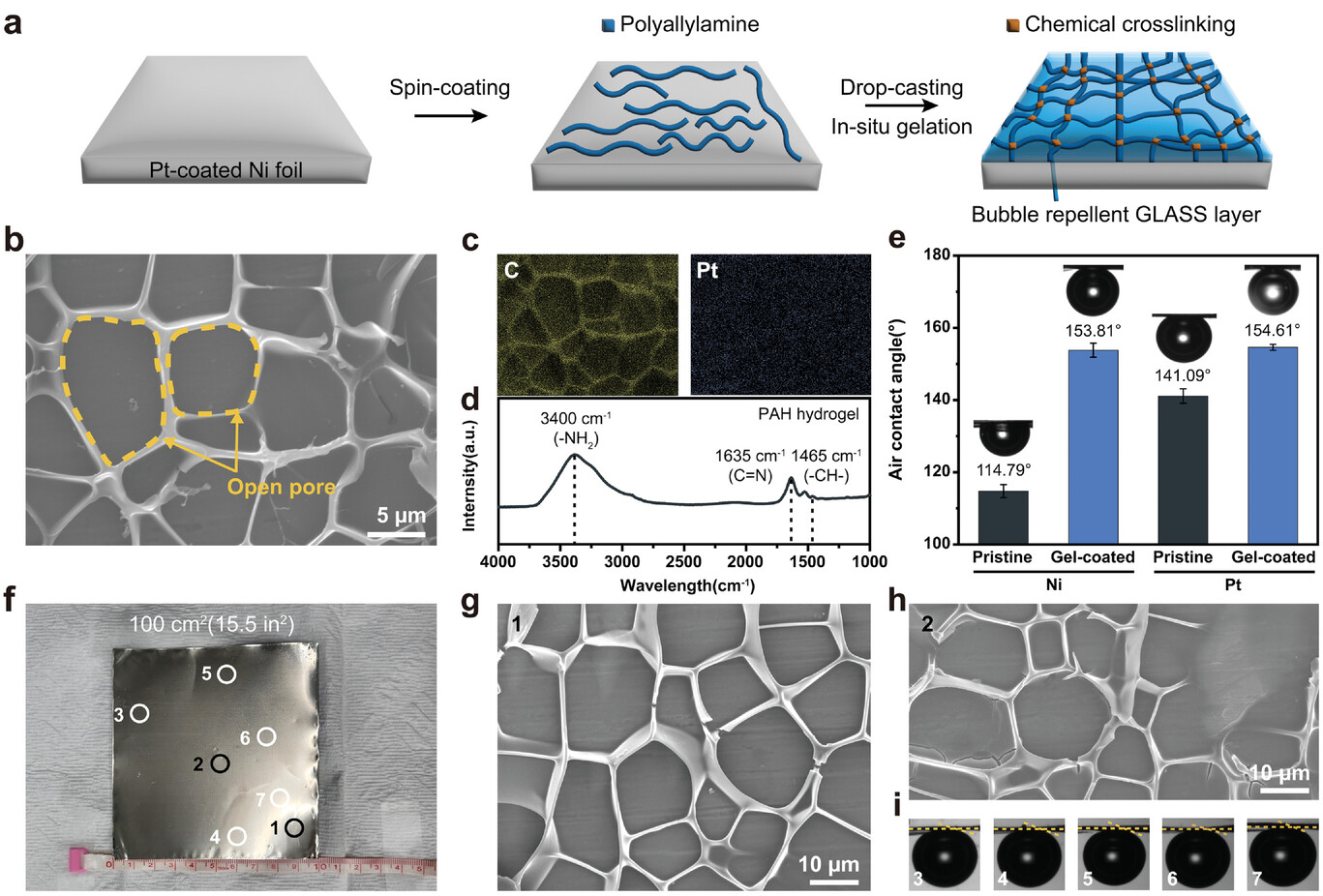
Figure 2. Morphology and aerophobicity of gel-coated electrodes. a) Experimental scheme for the fabrication of PAH hydrogel-coated electrodes. b) Top-view SEM image of the gel-coated Pt electrode. c) Elemental mapping images of gel-coated Pt electrodes: yellow for C and blue for Pt. d) FT-IR spectrum of the gel-coated Pt electrode. e) Static air contact angles of Ni and Pt electrodes before and after coating with PAH hydrogels at 1400 rpm. f) Photograph of the large-scale fabrication of gel-coated Pt electrode (100 cm2). g–i) Uniform coating of bubble-repellent PAH hydrogels on a large area was confirmed by (g, h) SEM and (i) static contact angle measurement. SEM and contact angles were measured at multiple locations, as shown in (f).
The application of the hydrogel-coated electrode system has yielded remarkable results. By expeditiously eliminating gas bubbles through the hydrophilic hydrogel coating, electrolytes could permeate the electrode promptly, thereby enhancing efficiency. The system achieved an impressive increase in gas production efficiency of up to 2.3 times when implemented with nickel and platinum catalysts, marking a significant advancement from 65.7 mA/cm² to 151.5 mA/cm².
Moreover, the prevention of gas accumulation on the electrode surface mitigates strain on the catalyst, reducing the risk of clumping or peeling and ensuring the long-term stability of the electrolytic system. This groundbreaking technology possesses the potential to revolutionize electrochemical gas generation reactions, particularly in water electrolysis, thus making a substantial contribution to the commercialization of green hydrogen.
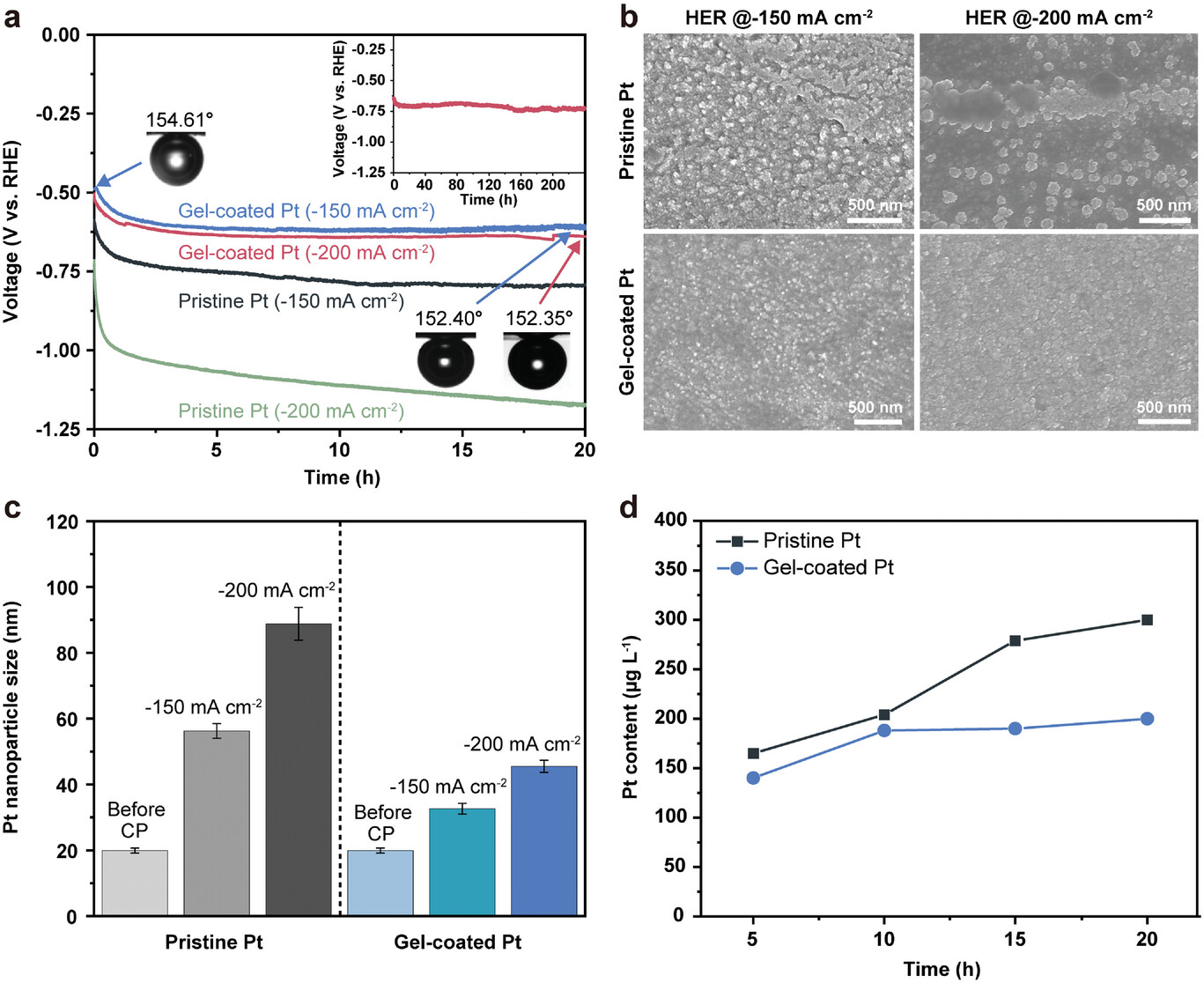
Figure 3, Stability of the pristine and gel-coated Pt electrodes for HER. a) Chronopotentiograms of the pristine and gel-coated Pt electrodes at −150 and −200 mA cm−2 for 20 h. The inset images show the air contact angles of the gel-coated electrodes before and after CP analysis for 20 h. The inset graph shows prolonged durability for 10 days. b) SEM images of the corresponding electrodes before and after the stability tests for 20 h. c) Size distribution of Pt nanoparticles before and after the stability tests for HER. d) Pt contents in electrolytes before and after the stability test at −150 mA cm−2.
“This hydrogel-based technology represents a creative approach to significantly enhance the efficiency of electrochemical gas generation reactions,” noted Professor Ryu. “By applying hydrogel, a commonly used material in everyday products such as wet bands, cosmetics, and diapers, to electrodes, we can drive advancements in water electrolysis reactions and expedite the commercialization of green hydrogen.”
Yunseok Kang (Combined MS/Ph.D. Program of Energy and Chemical Engineering at UNIST), the first author of this study, played a vital role in this research endeavor. The findings of this groundbreaking study have been published in Advanced Functional Materials on September 29, 2023. This research project was made possible through the support of the Mid-Career Researcher Program, the Technology Development Program to Solve Climate Changes, and the Regional Leading Research Center (RLRC) project, funded by the National Research Foundation of Korea (NRF) and the Ministry of Science and ICT (MSIT).
Journal Reference
Yunseok Kang, Seunghyun Lee, Seongsoo Han, et al., “Versatile, Stable, and Scalable Gel-Like Aerophobic Surface System (GLASS) for Hydrogen Production,” Adv. Funct. Mater., (2023).