A series of groundbreaking advancements in materials have recently emerged, promising a significant boost in the performance of redox flow batteries. These developments are anticipated to serve as a cornerstone for the development of new and innovative battery technologies.
In a groundbreaking achievement, Professor Hyun-Wook Lee from the School of Energy and Chemical Engineering at UNIST, in collaboration with Professor Dong-Hwa Seo from KAIST, has unlocked new possibilities by introducing stable ligands into oxidation-reduction materials. Their pioneering research significantly improves redox flow battery performance and presents cost-effective alternatives to conventional vanadium-based systems.
Redox flow batteries are highly regarded as large-scale energy storage devices due to their scalability, safety features, and flexibility. However, challenges such as limited global reserves and price volatility associated with vanadium have hindered its widespread use in these batteries. Additionally, traditional vanadium-based systems suffer from low operating voltage and slow oxidation-reduction reaction rates that limit overall battery performance improvements.
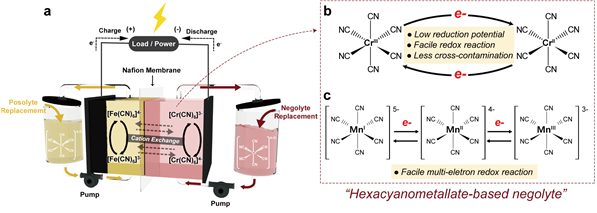
Figure 1. (a) Cell configuration and d-orbital splitting for different chromium octahedral complexes and (b) Scheme of the multielectron reaction of hexacyanomanganate ions.
To address these challenges head-on, the research team explored utilizing an octahedral “hexacyanometalate” structure—a transition metal ion complexed with six cyanide ligands (CN-)—to achieve exceptional electrochemical properties. In a groundbreaking approach, they proposed hexacyanometalate as an anode electrolyte material—a novel innovation that greatly enhances stability.
“We recognized the potential of redox flow batteries for energy storage but faced limitations when scaling up due to high unit prices associated with vanadium,” explained Professor Lee.
The researchers successfully employed hexacyanometalates in two types of redox flow cells: iron-chromium and iron-manganese configurations. Notably impressive is the fact that even after more than 500 charge-discharge cycles, the iron-chromium system maintained a high coulomb efficiency of over 99%. It achieved a voltage of 1.5V or higher, resulting in an energy density (38.6 Wh L−1) that surpassed existing redox flow cells by 30%.
In the iron-manganese redox flow battery, hexacyanomanganate was utilized as the anode electrolyte—a breakthrough that enabled a “two-electron reaction” and enhanced energy density compared to single-electron reactions at the same concentration. Raman analysis confirmed the smooth progression of the two-electron reaction during charging and discharging processes. The stability of this system was validated through more than 100 repeated charge-discharge cycles.
“This study showcases exceptional performance among chromium-based redox flow batteries reported to date,” stated Ji-Eun Jang, first author of the study from the Combined MS/PhD Program in Energy and Chemical Engineering at UNIST.
The study findings have been published ahead of their official publication in the online version of Advanced Energy Materials and ACS Energy Letters on July 7 and August 8, 2023, respectively. This work has been supported by the 2020 Research Fund of UNIST, Individual Basic Science & Engineering Research Program through National Research Foundation of Korea funded by the Ministry of Science and ICT (MSIT), as well as the Korea Institute of Energy Technology Evaluation and Planning (KETEP). These groundbreaking findings pave the way for further advancements toward cost-effective redox flow batteries with enhanced performance characteristics—bringing us one step closer to realizing efficient large-scale energy storage solutions.
Journal Reference
Ji-Eun Jang, Ryeong-ah Kim, S. Jayasubramaniyan, et al., ‘Full-Hexacyanometallate Aqueous Redox Flow Batteries Exceeding 1.5 V in an Aqueous Solution,’ Adv. Energy Mater., (2023).
Journal Reference
Ji-Eun Jang, S. Jayasubramaniyan, Seok Woo Lee, and Hyun-Wook Lee, ‘A Hexacyanomanganate Negolyte for Aqueous Redox Flow Batteries,’ ACS Energy Lett., (2023).