A research team led by Professor Hyun-Wook Lee in the School of Energy and Chemical Engineering at UNIST has identified the causes of oxygen generation in a novel cathode material called quasi-lithium and proposed a material design principle to address this issue.
Quasi-lithium materials theoretically enable batteries to store 30% to 70% more energy compared to existing technologies through high-voltage charging of over 4.5V. This advancement could allow electric vehicles to achieve a driving range of up to 1,000 km on a single charge. However, during the high-voltage charging process, oxygen (O-2) trapped inside the material can oxidize and be released as gas (O2), posing a significant explosion risk.
The research team discovered that oxygen oxidizes near 4.25V, causing partial structural deformation and gas release. They proposed a novel electrode material design aimed at fundamentally preventing the oxidation of oxygen by substituting some of the transition metals in quasi-lithium with elements that have lower electronegativity.
Due to the difference in electronegativity between the two metal elements, electrons accumulate around the more electronegative element, increasing the availability of electrons for the transition metal and preventing oxidation of oxygen. Conversely, when there are insufficient available electrons in the transition metal, the oxygen substitutes and releases electrons, resulting in its oxidation and gas emission.
First author Min-Ho Kim, a PhD researcher at UNIST and a postdoctoral researcher at UCLA, explained, “While previous studies focused on stabilizing oxidized oxygen to prevent its gas emission, our research differentiates itself by addressing the prevention of oxygen oxidation itself.”
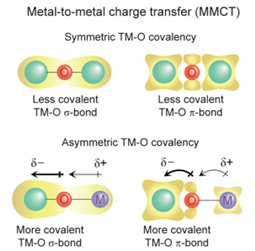 Figure 1. Illustrations of symmetric TM─O covalency in TM-O-TM configuration and proposed asymmetric TM─O covalency in TM-O-M configuration. In contrast to TM-O-TM configuration, asymmetric covalency in TM-O-M induces nonequivalent electron distribution toward more electronegative group.
Figure 1. Illustrations of symmetric TM─O covalency in TM-O-TM configuration and proposed asymmetric TM─O covalency in TM-O-M configuration. In contrast to TM-O-TM configuration, asymmetric covalency in TM-O-M induces nonequivalent electron distribution toward more electronegative group.
Additionally, this change in electron density can induce a rise in charging voltage, leading to the achievement of high energy density. Since energy density is proportional to the number of available electrons and the charging voltage, the strategy of substituting transition metals ultimately enables more energy storage per unit weight of the battery. This principle is akin to how a dam can store more energy the more water it has and the greater the height of the fall.
The research team experimentally confirmed the oxygen oxidation suppression effect of the transition metal (TM) substitution. X-ray analysis conducted with an accelerator showed that substituting part of ruthenium with nickel significantly reduced oxygen gas emissions. Theoretical validation of charge redistribution was achieved through density functional theory (DFT) calculations.
Professor Lee stated, “Through various experiments and theoretical analyses, we have developed a library of techniques that can guide cathode material researchers in their material development efforts. This work will contribute to the development of explosion-free long-range batteries with increased energy density.”
This research was supported by the National Research Foundation of Korea (NRF)’s International Cooperation Development Project for Fundamental Technology and was published online on February 19 in Science Advances, a sister journal of Science, published by the American Association for the Advancement of Science (AAAS).
Journal Reference
Min-Ho Kim, Haeseong Jang, Eunryeol Lee, et al., “Metal-to-metal charge transfer for stabilizing high-voltage redox in lithium-rich layered oxide cathodes,” Sci., Adv., (2025).












