Ionizing radiation (IR) therapy is frequently used in the treatment of cancer and is believed to exert its effects by DNA breaks in cells. Radiation from heavy charged particles such as carbon ions produced by particle accelerators has a high linear energy transfer (LET) and releases most of its energy within a short range, called the Bragg peak. If the Bragg peak is focused on the tumor, damage to surrounding normal tissues can be minimized compared to the commonly used low LET radiation such as gamma- or x-rays (Figure 1A).
DNA double-strand breaks (DSBs) generated by high LET heavy ion radiation (hiLET-DSBs) are more “complex” than low LET-induced DSBs (loLET-DSBs), as they carry additional DNA damage such as apurinic/apyrimidinic (AP) site and thymine glycol (Tg) in close proximity to the DSB sites, making it more cytotoxic per unit dose than low LET radiation (Figure 1B). It has not been fully investigated how these hiLET-DSBs are processed in mammalian cells.
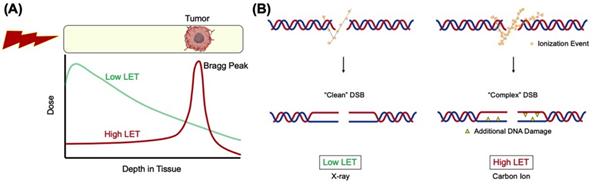
Figure 1. (A) Depth dose distribution for low LET x-rays and monoenergetic Bragg curve for high LET carbon ions. (B) Carbon ions produce more “complex” DSBs and x-rays produce relatively “clean” DSBs.
The research team visited the QST hospital in Japan to use the synchrotron named HIMAC (Heavy Ion Medical Accelerator in Chiba) for high LET radiation. A similar synchrotron has been installed at Yonsei University and another one is scheduled to be installed at Seoul National University Hospital in Kijang in 2027. Dr. Takata’s research team intends to help establish a basic research program using these synchrotrons in Korea to improve heavy ion therapy.
Dr. Takata’s research team hypothesized that DNA polymerase θ (POLQ) is an important factor to repair complex DSBs. POLQ is a unique DNA polymerase that is able to perform microhomology-mediated end-joining as well as translesion synthesis (TLS) across an abasic (AP) site and thymine glycol (Tg). However, the biological significance of the TLS activity was unknown.
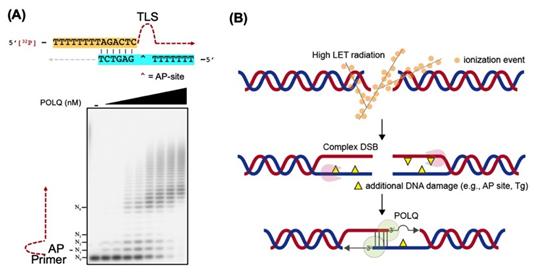
Figure 2. (A) POLQ is able to anneal two single-stranded DNA tails utilizing a short homology sequence and is able to bypass DNA damage. (B) A model of POLQ-mediated repair following high LET radiation. POLQ promotes synapsis formation of the two resected 3′-single-stranded DNA tails and efficiently bypasses DNA damage located on the tails.
Ms. Yubin Sung, one of the joint first authors, expounds “We provided evidence that the TLS activity of POLQ plays a critical role in repairing hiLET-DSBs. We found that POLQ efficiently anneals and extends substrates mimicking complex DSBs” (See Figure 2).
“We demonstrated that genetic disruption of POLQ results in an increase of chromatid breaks and enhanced cellular sensitivity following treatment with high LET radiation”, explains Mr. Geunil Yi, another joint first author (See Figure 3).
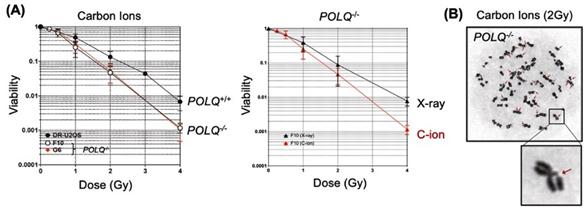
Figure 3. (A) Effect of POLQ deletion on cell survival fraction after carbon ion or x-ray irradiation. (B) POLQ deletion increases chromatid breaks after carbon ion irradiation.
The single-molecule FRET assay system to monitor POLQ-mediated annealing and DNA extension was developed in collaboration with Prof. Hajin Kim and Mr. Chanwoo Kim at UNIST. Ms. Jae Sun Ra at IBS-CGI analyzed chromatid breaks induced by high LET radiation. Prof. Fujimori and Mr. Hirakawa at QST, and Prof. Kato at Colorado State University helped conduct the experiments with HIMAC.
“We are proud to announce the publication of our paper which was only possible through the great teamwork of everybody involved,” noted Professor Takata. “Our findings provide new insights into the mechanisms of how hiLET-DSB is repaired in mammalian cells and further suggest that the inhibition of POLQ may augment the efficacy of heavy ion radiation therapy.”
Their findings have been published in the journal, Nucleic Acids Research on February 20, 2023.
Journal References
Geunil Yi, Yubin Sung, Chanwoo Kim, et al. “DNA polymerase θ-mediated repair of high LET radiation-induced complex DNA double-strand breaks,” Nucleic Acids Research (2023).












