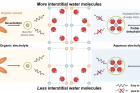
Sodium (Na)-ion batteries are rapidly gaining attention as the next-generation solution to replace lithium-ion batteries due to their lower production cost and similar battery systems. In a significant breakthrough, Professor Hyun-Wook Lee and his research team in the School of Energy and Chemical Engineering at UNIST has uncovered crucial factors affecting the performance and lifespan of sodium-ion batteries using Prussian blue analogues (PBAs) as cathode materials.
The research focused on understanding the influence of interstitial water molecules within PBA channels—a topic that had not been adequately explored until now. By conducting a comparative analysis of PBAs in aqueous and organic electrolytes, the study revealed that water molecules can hinder the insertion of hydrated Na+-ion depending on their quantity. Consequently, CuHCFe-1.4H2O with fewer interstitial water molecules demonstrated higher specific capacity in an aqueous electrolyte compared to CuHCFe-1.8H2O, which contained a higher number of interstitial water molecules.
Furthermore, it was discovered that these interstitial water molecules obstructed Na+ ion diffusion, resulting in poor kinetic properties for sodium-ion battery operation. The identification of these novel roles played by interstitial water molecules provides valuable insights for designing high-performance PBAs specifically tailored for sodium-ion battery cathodes.
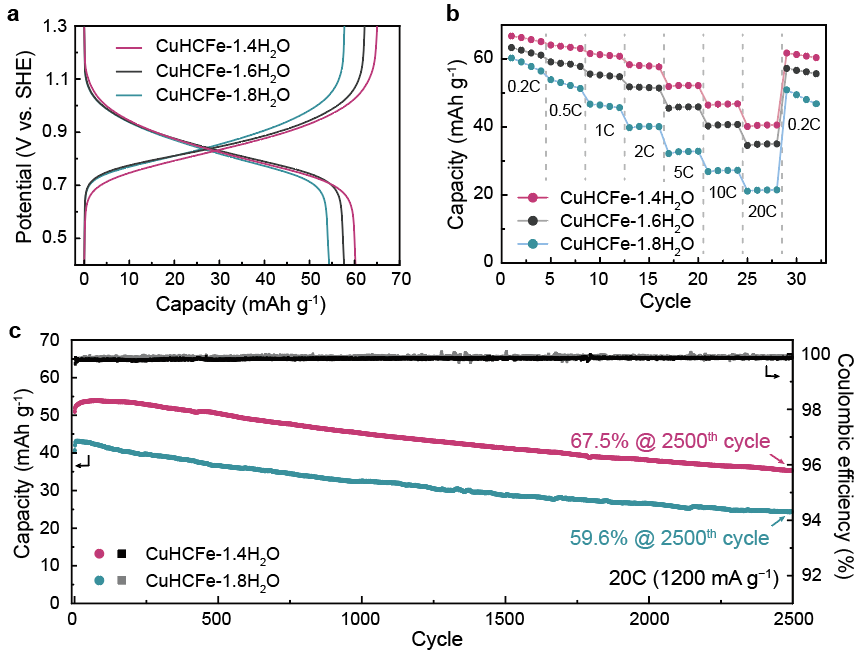
Figure 1. Electrochemical characteristics of CuHCFe samples in an aqueous electrolyte.
PBAs are a highly promising cathode material for Na+ ion batteries, offering advantages such as large channel size and stability in both aqueous and organic electrolytes. However, the impact of interstitial water molecules within PBA channels on battery performance has been insufficiently explored. In this study, the research team demonstrated that the quantity of water molecules can hinder the insertion of hydrated Na+ ions in PBAs, thereby affecting their overall electrochemical performance when compared in aqueous and organic electrolytes.
The experimental results confirmed that anode materials with fewer interstitial water molecules exhibited higher energy efficiency and prolonged lifespan. Notably, a reduction of approximately 24% in the number of water molecules led to a 9.7% improvement in battery capacity, while maintaining 67.5% capacity after undergoing 2,500 charge/discharge cycles. Conversely, cathode materials with a larger quantity of interstitial water molecules showed only 59.6% capacity retention under the same experimental conditions.
Additionally, this reduction (24%) in water molecule content resulted in sodium ions being activated over four times more frequently within the aqueous electrolyte system—effectively doubling the battery’s speed.
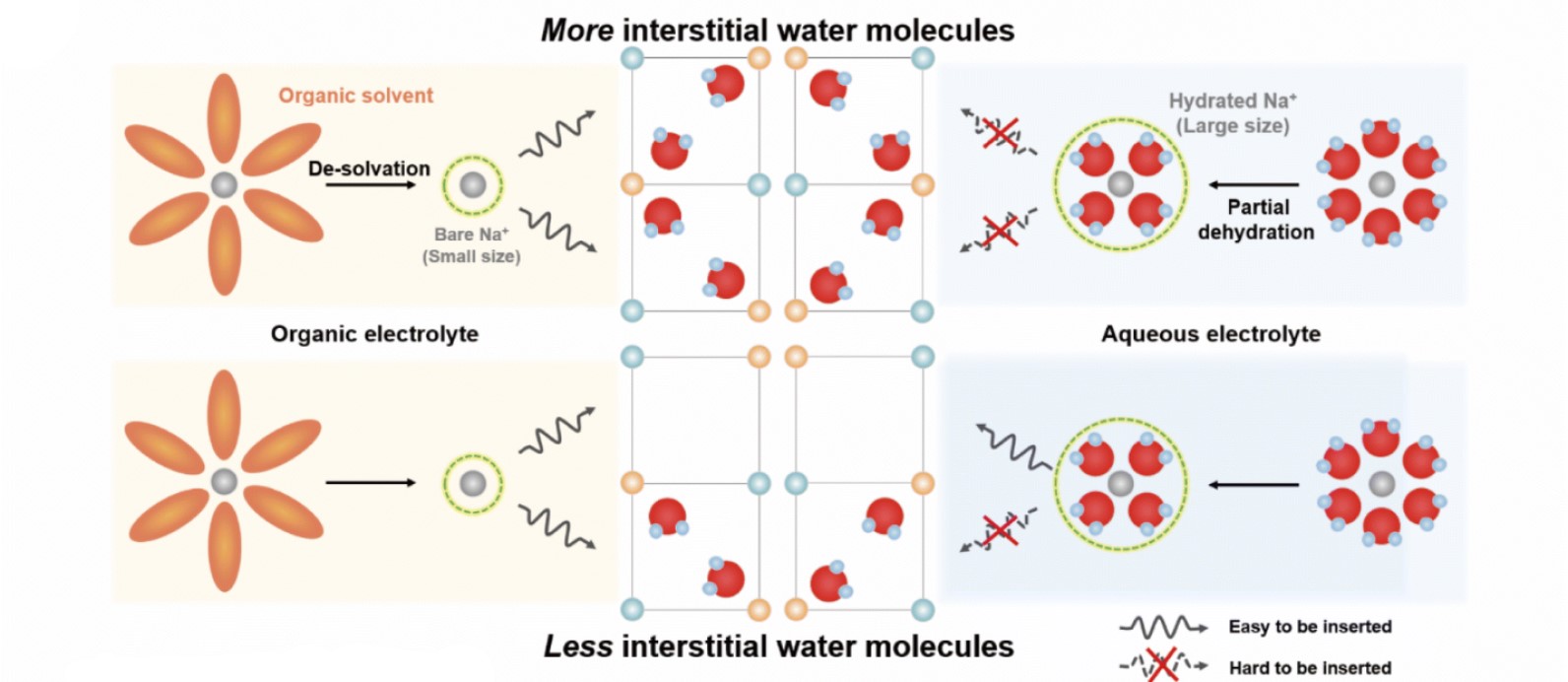
Figure 2. Electrochemical properties of CuHCFe in an organic electrolyte and the different mechanisms of CuHCFe in aqueous and organic electrolytes. Above is the schematic illustration of the different behaviors of Na+ ions in organic and aqueous electrolytes. The different mechanisms suggest that hydrated Na+ ions play a significant role in governing intercalation and deintercalation into PBAs.
Professor Lee emphasized the need for further research on capacity-induced factors, such as Prussian blue and Prussian white. He highlighted that “[U]nderstanding these underlying factors, which are yet to be fully comprehended, is critical for developing high-performance sodium secondary batteries.”
The study findings have been published ahead of their official publication in the online version of the Journal of Materials Chemistry A—a prestigious international academic journal specializing in energy and materials research—on May 29, 2023. This work has received support from the 2023 Research Fund of UNIST and Individual Basic Science and Engineering Research Program through the National Research Foundation (NRF) of Korea, funded by the Ministry of Science and ICT (MSIT).
Journal Reference
Donghyeon Kim, Ahreum Choi, Changhyun Park, et al., “Investigating the role of interstitial water molecules in copper hexacyanoferrate for sodium-ion battery cathodes,” J. Mater. Chem. A., (2023).