A technique to control the direction and rate of transport of colloidal particles in solution has been developed, using the partial concentration difference of salt occurs in salt water. As the operation required of them is very simple and no external power supply is necessary, it is expected to provide efficient environmental monitoring, such as pollutant field analysis in less developed countries.
Professor Taesung Kim and his research team in the School of Mechanical Aerospace and Nuclear Engineering at UNIST presented a dynamic transport control mechanism for colloidal particles by developing a micro-/nanofluidic DP platform (MNDP). This device can be easily applied to select specific components from cells mixed with fine particles of various sizes, or to identify contaminant components, and to synthesize quantum dot materials used for semiconductors and select only the same size. have. The advantage is that it operates purely from forces that differ from electrolyte ion concentrations.
The range of applications for controlling microparticles of various sizes ranging from nanometers (nm, 1 millionth of a meter) to micrometers (μm, one millionth of a million) is increasing. As a result, there have been many technologies for dealing with fine particles freely. Until now, it was difficult to apply them directly in the field because an external device such as a high-pressure environment or an electronic device was needed.
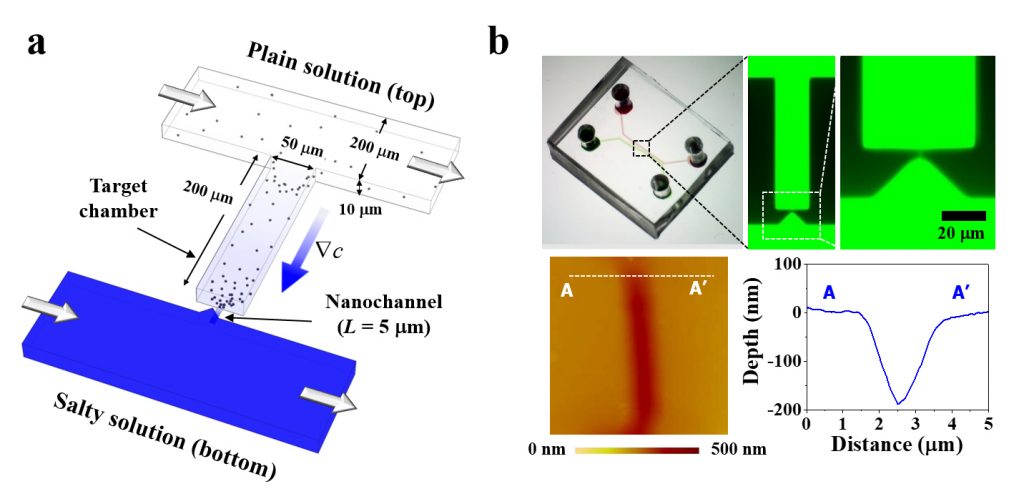
Figure 1. Concept, fabrication, and working mechanism of the hybrid-scale micro-/nanofluidic DP platform (MNDP). (a) Schematic of the MNDP in the production of a stable concentration gradient in the target chamber through a nanochannel. (b) Microscopic images of the MNDP filled with 1 mM of fluorescence dye.
In this study, the microfluidic device is a “diffusiophoresis” technology that controls the movement of charged microparticles by naturally regulating the forces generated by differences in electrolyte ions. In diffusiophoresis technology, microparticles are powered by osmotic pressure and electric field. Osmotic pressure always moves the fine particles in the solution from a low concentration of ions to a high one, but the direction of moving the fine particles by the electric field changes depending on the type of electrolyte used.
For example, in the case of salt (NaCl), the diffusion rate of anion (Cl-) is fast, so that the place where the ion concentration is relatively low is the cathode, and the place where the concentration is high becomes the anode. Move to place (anode). On the other hand, when an electrolyte with a fast cation diffusion rate is used, the direction of the electric field is reversed, which causes the force to move the fine particles from the low ion concentration to the high (cathode).
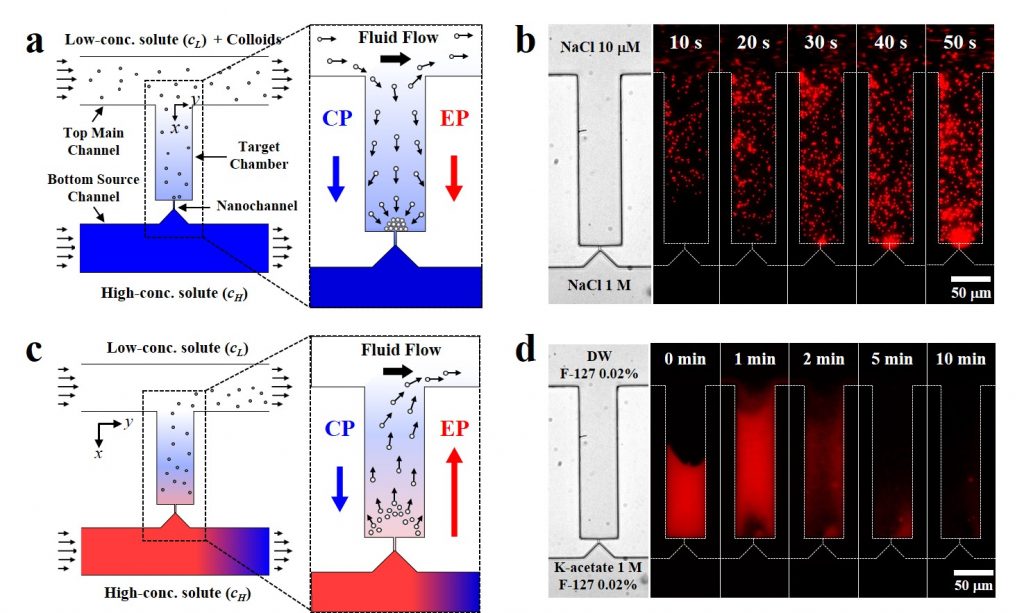
Figure 2. Simple and on-demand extraction of the preconcentrated colloidal particles.
The team developed a microfluidic device that can effectively control these two forces created by the difference in electrolyte ion concentrations, using nanochannels with diameters that can only pass ions. If the osmotic pressure acting on the microparticles in the microfluidic device and the direction of the force due to the electric field are the same, the particles are concentrated. If the two forces are opposite, the concentrated microparticles are extracted by the electric force.
Using this microfluidic device, the microparticles of 1 micrometer-sized negative charge inside the brine were successfully concentrated to 300 times the concentration for 1 hour. In addition, by changing the type of electrolyte and reversing the direction of the electric field, the concentrated microparticles could be extracted within 10 minutes.
“Since the effect of the electric field and osmotic pressure varies depending on the size of the fine particles, it is possible not only to concentrate and extract one type of particle, but also to effectively separate fine particles of different sizes,” says Dogyeong Ha in the PhD program of Mechanical Engineering at UNIST, the first author of the study.
“Our study is significant academically in that it is the result of experimentally proved diffusion phenomena that finely regulates electrolyte ions to control the movement of microparticles,” says Professor Kim. “This device is easy to manufacture and operate and requires no external power, so it can be applied to the diagnosis of environmental conditions directly in the harsh environment or in developing countries.”
The findings of this research have been published in ACS Nano on October 10, 2019. This study has been supported by the National Research Foundation of Korea (NRF) through the Korean government (MSIT). All microfabrication processes were performed at the UNIST Central Research Facility Center.
Journal Reference
Dogyeong Ha, Sangjin Seo, Kyunghun Lee, and Taesung Kim, “Dynamic Transport Control of Colloidal Particles by Repeatable Active Switching of Solute Gradients,” ACS Nano, (2019).