A joint research team, led by Professor Yong Hong Kim and Dr. Suk Min Kim in the School of Energy and Chemical Engineering and the Graduate School of Carbon Neutrality at UNIST, along with Professor Hyung Ho Lee from the Department of Chemistry at Seoul National University, has achieved a groundbreaking advancement in enzyme engineering. Their research focuses on redesigning Ni–Fe carbon monoxide dehydrogenases (CODHs) to overcome the challenge of oxygen (O2) sensitivity, a critical limitation in industrial applications.
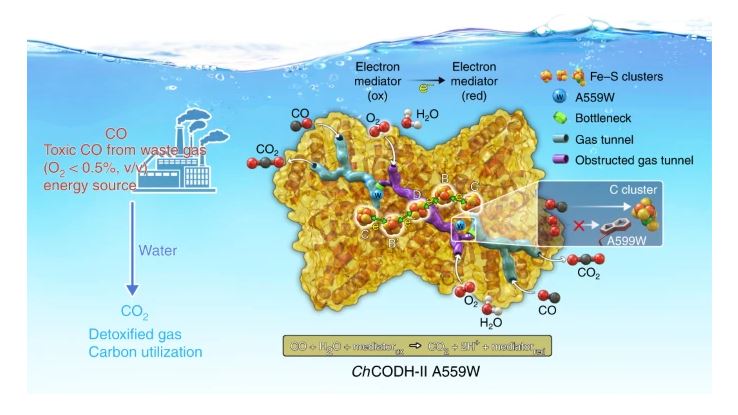
Figure 1. Tunnel-redesigned CO dehydrogenase. Substituting one residue in a predicted tunnel within CO dehydrogenase-II from Carboxydothermus hydrogenoformans (ChCODH-II) from Ala to Trp can decrease its O2 sensitivity without altering overall catalytic activity.
By comparing the structures of two CODH variants, ChCODH-II and ChCODH-IV, the team identified bottleneck residues and engineered a variant (A559W) with reduced oxygen sensitivity and maintained high turnover rates. Molecular dynamics simulations supported their findings, revealing obstruction in a gas tunnel due to a locked position of the mutated side chain.
The engineered enzyme demonstrated exceptional stability and efficiency when exposed to various gas mixtures, including high O2 levels and real flue gas from a steel mill. This advancement opens up possibilities for utilizing carbon-rich gases from industries, like steel without pretreatment.
Moreover, the enzyme was successfully employed in the CO hydration reaction, leading to formic acid production at scale. This breakthrough not only benefits the steel industry, but also offers potential in utilizing gases from coal and plastic waste as valuable resources.
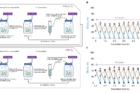
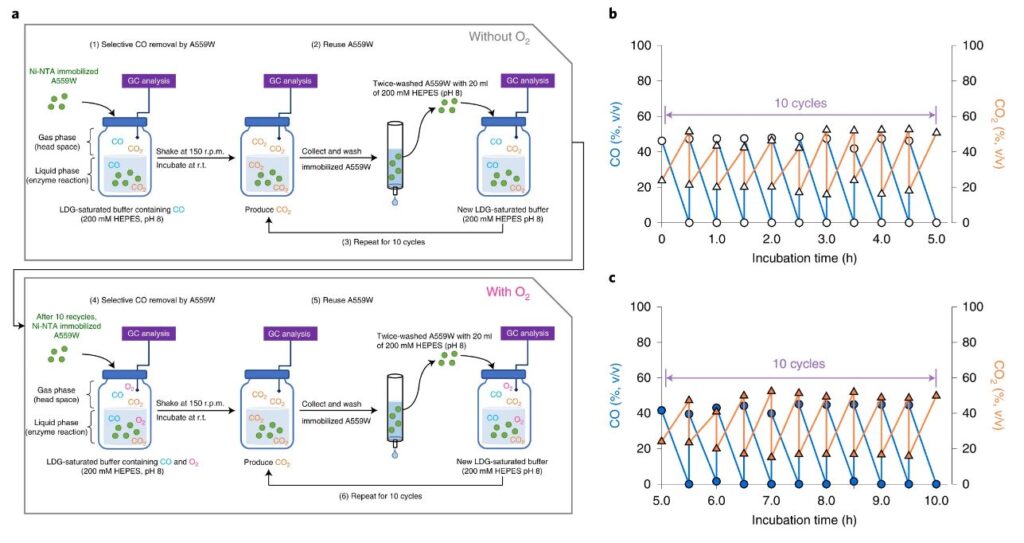 Figure 2. (a) A schematic for the experimental procedure, showing the repeated reuse of immobilized A559W. (b) Reusability of immobilized ChCODH-II A559W using LDG. (c) Reusability of immobilized ChCODH-II A559W using LDG with O2 (closed symbols).
Figure 2. (a) A schematic for the experimental procedure, showing the repeated reuse of immobilized A559W. (b) Reusability of immobilized ChCODH-II A559W using LDG. (c) Reusability of immobilized ChCODH-II A559W using LDG with O2 (closed symbols).
Professor Kim emphasized the practical validation of the enzyme at Hyundai Steel and POSCO sites, highlighting its role in advancing steel decarbonization initiatives. He stated, “The developed enzyme has successfully underwent direct validation at both facilities, playing a crucial role driving steel decarbonization endeavors.”
The research, supported by the C1 Gas Refinery Program and the Engineering Research Center Program through the Society of Science and ICT (MSIT) and the National Research Foundation of Korea (NRF), has been recognized in prestigious publications. The findings have been featured on the cover of Nature Catalysis, as well as highlighted in Nature and featured in a collection in Nature Chemical Engineering in 2022 and 2023, respectively.
Journal Reference
Suk Min Kim, Jinhee Lee, Sung Heuck Kang, et al., “O2-tolerant CO dehydrogenase via tunnel redesign for the removal of CO from industrial flue gas,” Nat. Catal., (2024).