In a pioneering discovery, researchers at UNIST have captured, for the first time, the elusive intermediate stages involved in the pairing of cell membrane proteins. Contrary to long-held beliefs that such proteins bind instantaneously, the study demonstrates that membrane protein interactions proceed through a zipper-like process, passing through multiple intermediate steps before forming a final, functional complex.
Led by Professor Duyoung Min in the Department of Chemistry, the research team used real-time single-molecule analysis to track the dynamic pairing process of membrane proteins embedded in cell membranes. Their findings reveal that proteins do not simply come together suddenly; instead, they engage gradually, initially connecting at specific sites and then progressing through several stages until a complete bond is formed.
This insight was made possible by a novel tool developed by the team, the ‘Single-Molecule Tweezers‘ technique that gently grips and manipulates individual proteins, allowing precise observation of how their interactions evolve over time. By applying this method, the researchers could directly visualize the step-by-step assembly process, a feat previously thought to be out of reach.
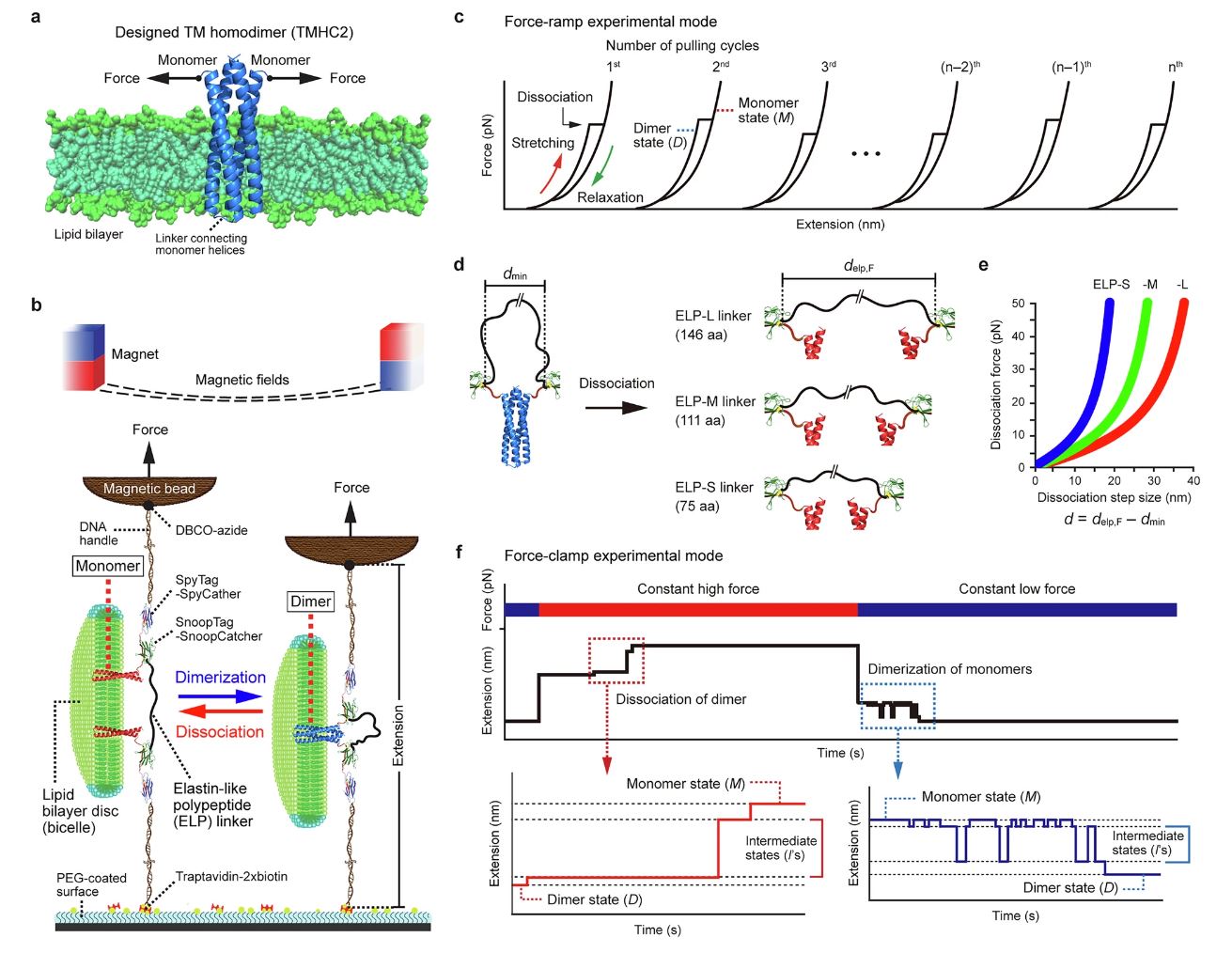
Figure 1. Schematic overview of their single-molecule tweezers and experimental modes.
Further experiments confirmed this mechanism. When the team introduced short peptide fragments designed to interfere with the protein-protein interaction, the pairing process stalled at the intermediate stage. Similar to a zipper that cannot fully close if a tooth is broken, the membrane proteins could not complete their binding when key steps were obstructed.
Professor Min emphasized the significance of these findings, “Understanding that membrane proteins assemble gradually through multiple steps marks a major turning point in our comprehension of cellular communication. Notably, this mechanism is relevant to targeted therapies—such as the breast cancer drug Perjeta, which inhibits protein pairings—and our ability to selectively block these hidden intermediate stages could lead to more precise and effective drug design.”
He further added, “The single-molecule tweezers approach used in this study has strong potential to uncover other fundamental processes in cell biology that are critical for medical research and drug development.”
The study, published in Nature Communications on August 9, 2025, was supported by the National Research Foundation of Korea and UNIST.
Journal Reference
Victor W. Sadongo, Eojin Kim, Seoyoon Kim, et al., “Single-molecule tweezers decode hidden dimerization patterns of membrane proteins within lipid bilayers,” Nat. Commun., (2025).












