A research team, led by Professor Youngkook Kwon in the Department of Energy and Chemical Engineering at UNIST has introduced a highly efficient and stable OER electrode by immobilizing the high conductive Ni3N particles on the V-incorporated NiFeOOH surface through an in situ electrochemical reconstruction method to overcome the above issues.
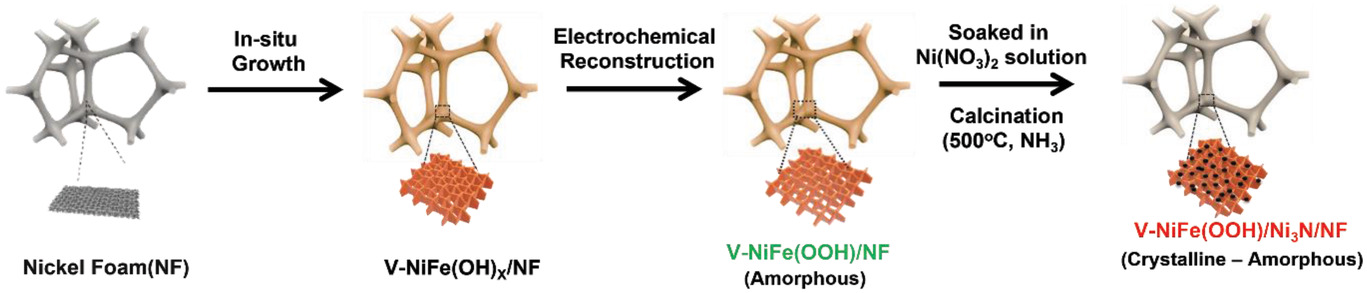
Figure 1. Schematic illustration of the fabrication of V-NiFeOOH/Ni3N/NF electrode.
Illustrated above is the two-step synthesis of V-NiFeOOH/Ni3N catalysts on a nickel foam (NF) electrode. In this study, the research team adopted the NF electrode for catalyst fabrication because industrial electrolyzers use Ni as current collectors. Moreover, NF possesses high electric conductivity, and its 3D porous macro- or microstructures enable favorable gas evolution properties at high current densities. As shown above, first the V-NiFeOOH/NF surface was prepared through in situ growth of V-NiFe(OH)x structures on the NF, followed by electrochemical reconstruction through continuous cyclic voltammetry (CV) cycling. Then, the as-prepared electrode was soaked in nickel nitrate solution and annealed in the presence of NH3 for 2 h at 500°C.
Water electrolysis technology is a representative technology that produces green hydrogen, a future energy carrier. The key here is the price of green hydrogen produced. The anion exchange membrane water electrolysis system is a system developed to increase hydrogen economy compared to the existing water electrolysis technology. However, it is still in the research stage, and in particular, in order to increase the durability of BOP (Balance of Plant), water electrolysis technology using ultrapure water must be developed. In the case of ultrapure electrolytes, it is more challenging to develop suitable catalysts because additional energy is required than aqueous electrolysis, which is acidic or alkaline.
The research team grew nickel nitride on the surface of vanadium-nickel-iron oxyhydroxide through electroplating and nitriding processes to manufacture a non-precious metal-based high-performance oxygen generation catalyst. Nickel-iron oxyhydroxides have the disadvantage of low electrical conductivity despite being a typical oxygen generation catalyst. To compensate for this, vanadium was doped and nickel nitride was grown on the surface to improve electrical conductivity, and at the same time, the active point was stabilized to ensure performance and long-term stability. Improved electrical conductivity accelerated the electron transfer rate at the interface between the catalyst and electrolyte and demonstrated an excellent reaction rate. The developed catalyst showed excellent performance not only in the alkaline environment but also in the anion exchange membrane water electrolysis system that flows ultrapure water.
The findings of this research have been published online ahead of print in Advanced Energy Materials and featured on the front cover on February 10, 2023. This study has been supported by the mid-sized research of the Korea Research Foundation, the Energy Technology Development Project of the Ministry of Trade, Industry and Energy, and the Creative Convergence Research Project of the National Science and Technology Research Association.
Journal Reference
Pandiarajan Thangavel, Hojeong Lee, Tae-Hoon Kong, et al., “Immobilizing Low-Cost Metal Nitrides in Electrochemically Reconstructed Platinum Group Metal (PGM)-Free Oxy-(Hydroxides) Surface for Exceptional OER Kinetics in Anion Exchange Membrane Water Electrolysis,” Adv. Energy Mater., (2022).












