As the world grapples with mounting energy demands and environmental crises, including extreme temperatures and heavy snowfall, the need for effective policies and sustainable solutions has become increasingly urgent. Amidst this pivotal moment, a research team led by Professor Youngkook Kwon in the School of Energy and Chemical Engineering at UNIST has unveiled a groundbreaking possibility—a solution that offers hope in addressing two pressing challenges simultaneously: the reduction of carbon dioxide (CO2), a potent greenhouse gas contributing to climate change, and harnessing nitrate (NO3−), a raw material responsible for fine dust pollution. This solution revolves around the synthesis of urea, an essential compound extensively utilized across various industries.
The key to this remarkable breakthrough lies in Professor Kwon’s pioneering application of cutting-edge technology for controlling atomic-level gaps within copper catalysts. By precisely manipulating these gaps on the atomic scale, he successfully enhances the catalytic performance necessary for efficient conversion processes.
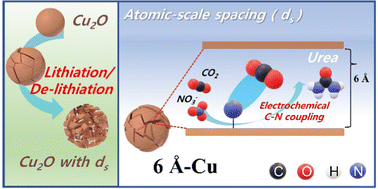
Figure 1. A brief overview of the study.
Urea plays an essential role in global food production as a nitrogen-based fertilizer that nourishes crops—contributing significantly to feeding over half of the world’s population. However, conventional methods of urea synthesis heavily rely on non-renewable energy sources while generating substantial amounts of CO2 emissions that exacerbate climate change concerns.
In response to these pressing issues, Professor Kwon, alongside a collaborative effort with Professor Hyun-Kon Song and Professor Hyun-Wook Lee in the School of Energy and Chemical Engineering at UNIST, has embarked on an extraordinary journey towards finding alternative approaches—ones that are environmentally friendly while maximizing resource utilization.
Using the lithiation approach, Professor Kwon’s team, in collaboration with Professor Song’s team, discovered that atomic-scale spacings (ds) between Cu facets greatly enhance the activity of CR-CO2/NO3− and selectivity for urea synthesis. To further validate their findings, Professor Lee employed real-time transmission electron microscopy (TEM) analysis. Through this advanced imaging technique, he observed and identified the formation of atomic-level gaps between both sides of copper nanoparticles—a crucial step towards achieving optimal catalytic performance.
Their findings have revealed that among the various copper catalysts generated during their research, the 6Å-Cu catalyst with a gap distance of 6 angstroms (Å) exhibited remarkable performance enhancements. Compared to existing copper catalysts, it achieved an impressive 17-fold increase in urea production rate (7541.9 μgh-1 mgcat-1) and a significant 19-fold increase in urea current density (115.25 mA cm-2)—the highest reported figures within academia to date.
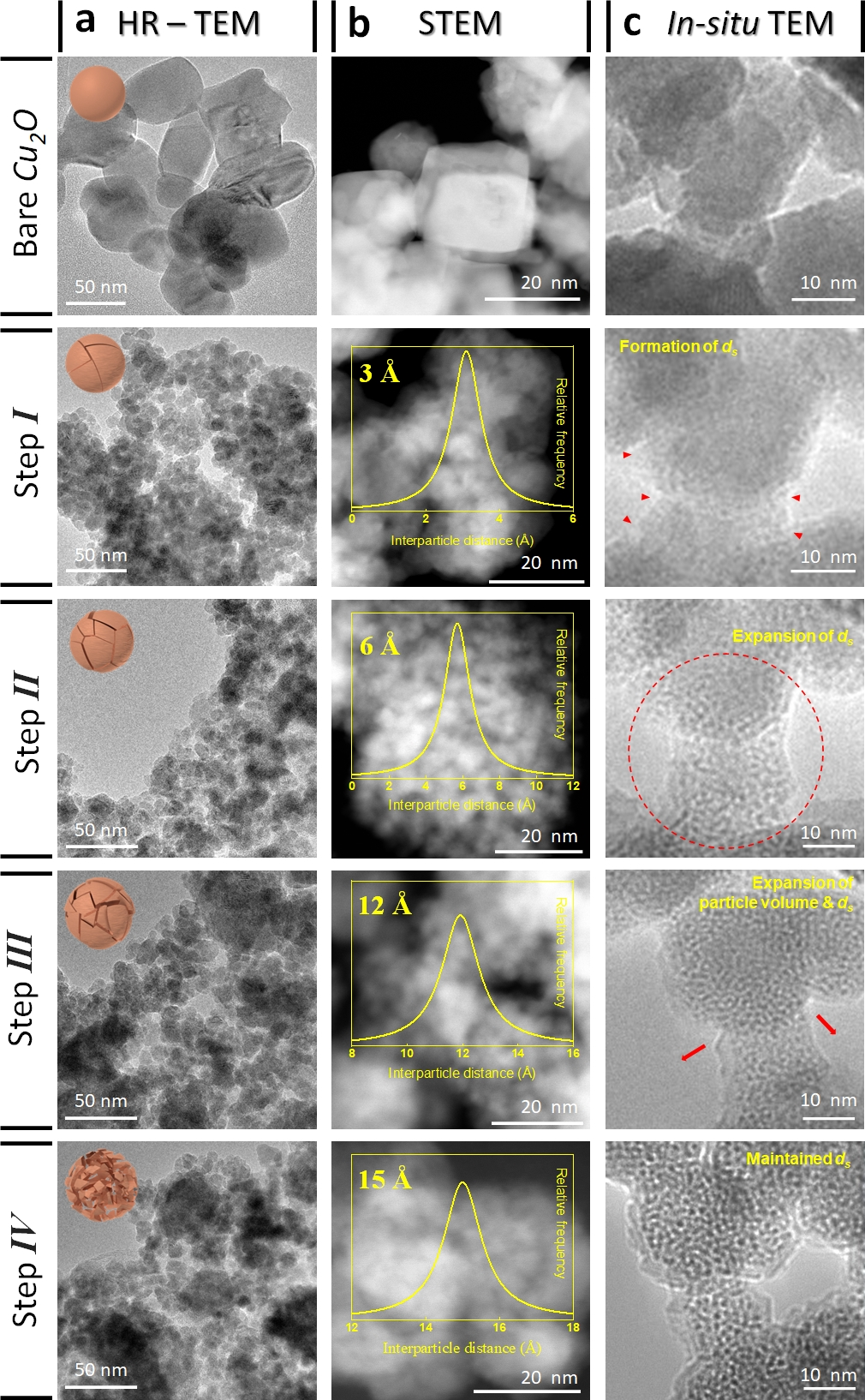
Figure 2. High-resolution transmission electron microscopy (HR-TEM), scanning transmission electron microscopy (STEM), and in situ TEM analysis of bare and lithiated Cu2O according to a degree of lithiation. (a) Ex situ HR-TEM images with their representative scheme. Structure and morphology evolution were described with the scheme on HR-TEM images. (b) STEM images of Cu2O with ds. The generated ds was observed from the HAADF-STEM image. The distribution of ds is described in each STEM image as an inset graph. (c) Time-lapse TEM images along lithiation progress were monitored. Structural changes of Cu2O during lithiation were investigated in real-time at 0.5 V bias by a TEM.
This research breakthrough offers a promising pathway for more efficient and sustainable synthesis of urea through electrochemical processes. By developing catalysts with controlled atomic-scale spacings, scientists can significantly enhance the yield and performance of urea production from CO2 and nitrate co-reduction reactions.
For further in-depth insights into this study, please refer to the original publication available at the provided link.
The study findings have been published ahead of their official publication in the online version of Energy & Environmental Science, a world-renowned journal in the field of energy and environmental science, on March 28, 2023. This work was selected for inclusion on the inside back cover of the journal and was subsequently published on May 17, 2023. The research received support from various institutions including the National Research Foundation of Korea (NRF), the Carbon Neutral Institute (CNI) at UNIST, and the Ministry of Trade, Industry and Energy (MOTIE).
Journal Reference
Seokmin Shin, Siraj Sultan, Zong-Xian Chen, et al., “Copper with an atomic-scale spacing for efficient electrocatalytic co-reduction of carbon dioxide and nitrate to urea,” Energy Environ. Sci., (2023).



















