If DNA bridges persist between chromosomes during cell division, it can lead to abnormal segregation, genetic instability, and potentially cancer. Researchers at UNIST and the Institute for Basic Science (IBS) have, for the first time, elucidated how a critical protein functions as a final safeguard to eliminate these dangerous DNA bridges—often operating at the very last moment of cell division.
Distinguished Professor Anton Gartner of the Graduate School for Health Sciences and Technology at UNIST and Associate Member of the IBS Center for Genomic Integrity, along with IBS Research Fellow Stephane Rolland, have uncovered the molecular mechanism by which the protein LEM-3 cleaves DNA bridges during cytokinesis. Their findings have been published in Nucleic Acids Research on April 11, 2025.
Cell division is a vital biological process that renews tissues and maintains organism health. In humans, billions of cells divide daily: intestinal cells are renewed every 1–3 days, and skin cells roughly every 2–3 weeks. During division, DNA must be accurately duplicated and separated. However, errors such as incomplete replication or chromosome entanglement can result in DNA bridges connecting daughter cells. If left unresolved, these bridges can cause chromosomal instability, loss of genetic information, and increase the risk of cancer.
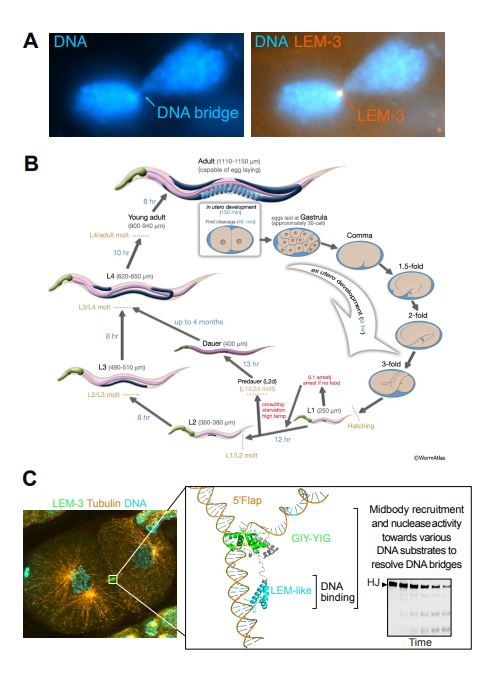 Figure 1. Functional analysis of the LEM-3 protein and the roles of its domains using the C. elegans model. (A) Evidence showing that LEM-3 accumulates at DNA bridges remaining between daughter cells during the final stages of cell division and contributes to their resolution. This was observed through fluorescence microscopy. (B) LEM-3 function was analyzed across various developmental stages using the C. elegans model, elucidating the mechanism by which DNA bridges are severed during cell division. (C) LEM-3 localizes to the midbody to recognize and cleave DNA bridges. Structural prediction and cleavage assays revealed that the LEM-like domain is responsible for DNA recognition, while the GIY-YIG domain directly mediates DNA cleavage.
Figure 1. Functional analysis of the LEM-3 protein and the roles of its domains using the C. elegans model. (A) Evidence showing that LEM-3 accumulates at DNA bridges remaining between daughter cells during the final stages of cell division and contributes to their resolution. This was observed through fluorescence microscopy. (B) LEM-3 function was analyzed across various developmental stages using the C. elegans model, elucidating the mechanism by which DNA bridges are severed during cell division. (C) LEM-3 localizes to the midbody to recognize and cleave DNA bridges. Structural prediction and cleavage assays revealed that the LEM-like domain is responsible for DNA recognition, while the GIY-YIG domain directly mediates DNA cleavage.
Previous research revealed that LEM-3 plays a crucial role in resolving persistent DNA bridges, acting as a last resort when other repair mechanisms fail. Notably, LEM-3 localizes to the midbody—the narrow structure connecting two daughter cells during the final stages of division. The absence of LEM-3 results in persistent DNA bridges and failed cell division, highlighting its importance in maintaining genomic integrity.
Although LEM-3 functions as a nuclease—acting like a molecular scalpel that cleaves DNA—its reaction mechanism, substrate specificity, and the roles of its various domains were not fully understood. The research team investigated how LEM-3 recognizes and cleaves different DNA structures, as well as how its domains contribute to its localization, catalytic activity, and DNA-binding ability. Additionally, they discovered that mislocalization of LEM-3 can be detrimental: if it improperly enters the nucleus, it can cause unintended DNA damage, ultimately leading to embryonic lethality. These findings highlight the critical importance of tightly regulating LEM-3 activity within cells to prevent harmful effects.
The study was conducted using Caenorhabditis elegans, a model organism whose LEM-3 protein is evolutionarily conserved as human ANKLE1. Professor Gartner remarked, “Given that ANKLE1 has been linked to breast and colorectal cancers, our findings may pave the way for new strategies in cancer prevention and treatment.”
This research was supported by the Ministry of Science and ICT (MSIT) through the National Research Foundation of Korea (NRF), the IBS, and the Biotechnology and Biological Sciences Research Council (BBSRC) of the United Kingdom.
Journal Reference
Junfang Song, Peter Geary, Khadisha Salemova, et al., “Functional dissection of the conserved C. elegans LEM-3/ANKLE1 nuclease reveals a crucial requirement for the LEM-like and GIY-YIG domains for DNA bridge processing,” Nucleic Acids Res., (2025).