A groundbreaking study, led by Professor Kyemyung Park and his research team in the Graduate School of Health Science and Technology and the Department of Biomedical Engineering at UNIST has shed light on the intricate mechanism behind the immune system’s ability to differentiate between self and non-self antigens. Their research, published in the esteemed journal Trends in Immunology, presents a novel quantitative framework that could pave the way for predictive models in immune-related disease treatment response.
The immune system is a complex network of cells and molecules that defends our bodies against pathogens while also preventing autoimmune diseases. Autoimmune diseases occur when the immune system mistakenly attacks its own cells and tissues, due to the recognition of self-antigens by self-reactive T cells. Although the existence of self-reactive T cells and their response to autoantigens has been observed, the mechanisms underlying the maintenance of self-tolerance have remained elusive.
In this study, the research team employed a multiscale systems biology and nonlinear dynamics approach to shed light on this intricate mechanism. By integrating individually published experimental findings, they proposed a novel concept called, the “Symmetry breaking between self- and non-self-antigens.”
According to this concept, continuous activation of self-reactive T cells is essential for maintaining equilibrium and self-tolerance through the activation of regulatory T cells, rather than promoting autoimmune diseases. The research team identified a threshold that maximizes the immune response of self-reactive T cells. Normally, self-reactive T cells are kept below this threshold through the selective action of regulatory T cells, while T cells responding to external antigens can surpass this threshold, leading to a normal immune response.
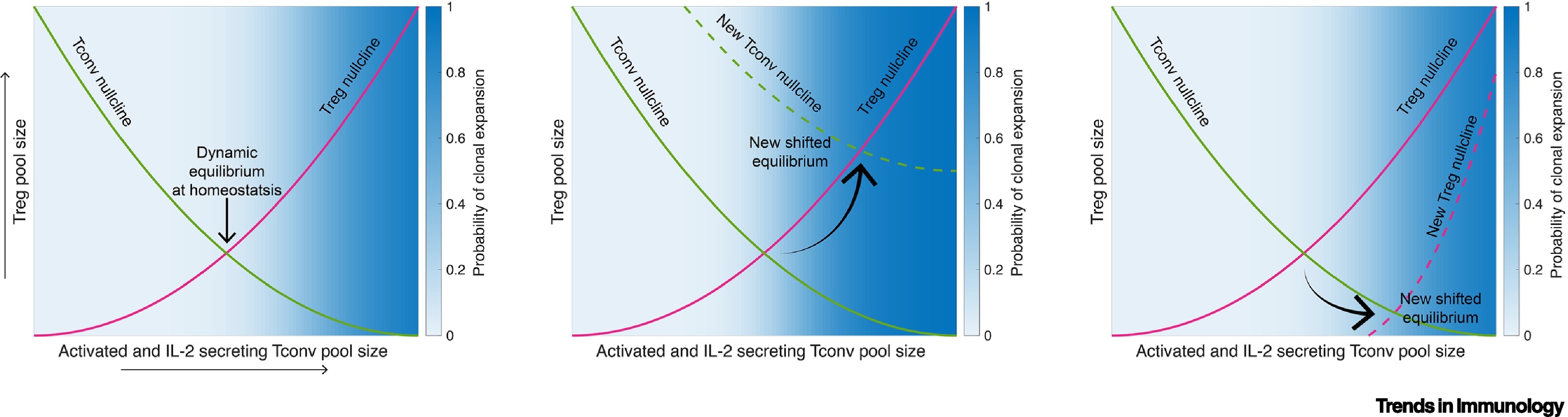 Figure 1. Illustration of how phase portraits corresponding to the dynamics of the Treg–Tconv interplay in different biological contexts can be helpful in understanding Treg behavior and function across contexts.
Figure 1. Illustration of how phase portraits corresponding to the dynamics of the Treg–Tconv interplay in different biological contexts can be helpful in understanding Treg behavior and function across contexts.
Professor Park highlighted the significance of their multiscale systems biology approach, stating, “By considering the interactions between cells, the dynamics occurring within the cells, and the spatial movement of cells, we were able to derive new perspectives that could not be obtained from individual experimental results.”
This research, conducted in collaboration with the National Institute of Health (NIH) and Yale Medical School, received support from various organizations, including the Ulsan Institute of Science and Technology, the Institute of Basic Science, the Korea Research Foundation, and the National Institute of Health (NIH).
The implications of this study are far-reaching, as it opens up new possibilities for predictive models in immune-related disease treatment response. With the rapid accumulation of immunological knowledge and related omics data, Professor Park envisions an era of precision medicine. Through the integration of systemic immunology, computational modeling, and artificial intelligence, complex immunological phenomena can be better understood and the immune system can be precisely controlled, leading to improved treatments and personalized approaches.
This groundbreaking research marks a significant step forward in immunology and sets the stage for future advancements in the field. As the understanding of the immune system continues to deepen, the potential for transformative breakthroughs in disease treatment and prevention becomes increasingly promising. Their findings have been published in the online version of the journal, Trends in Immunology on September 8, 2023.
Journal Reference
Shubham Tripathi, John S. Tsang, and Kyemyung Park, “Systems immunology of regulatory T cells: can one circuit explain it all?,” Trends Immunol., (2023).