A groundbreaking study conducted by Professor Jiyoung Park and her research team in the Department of Biological Sciences at UNIST has identified FAM3C, a metabolism-regulating signaling molecule produced by cancer-associated adipocytes (CAAs), as a key regulator of breast cancer progression within the tumor microenvironment (TME). The findings, published in the prestigious academic journal Cancer Research, shed light on the potential for targeted therapies in the treatment of breast cancer.
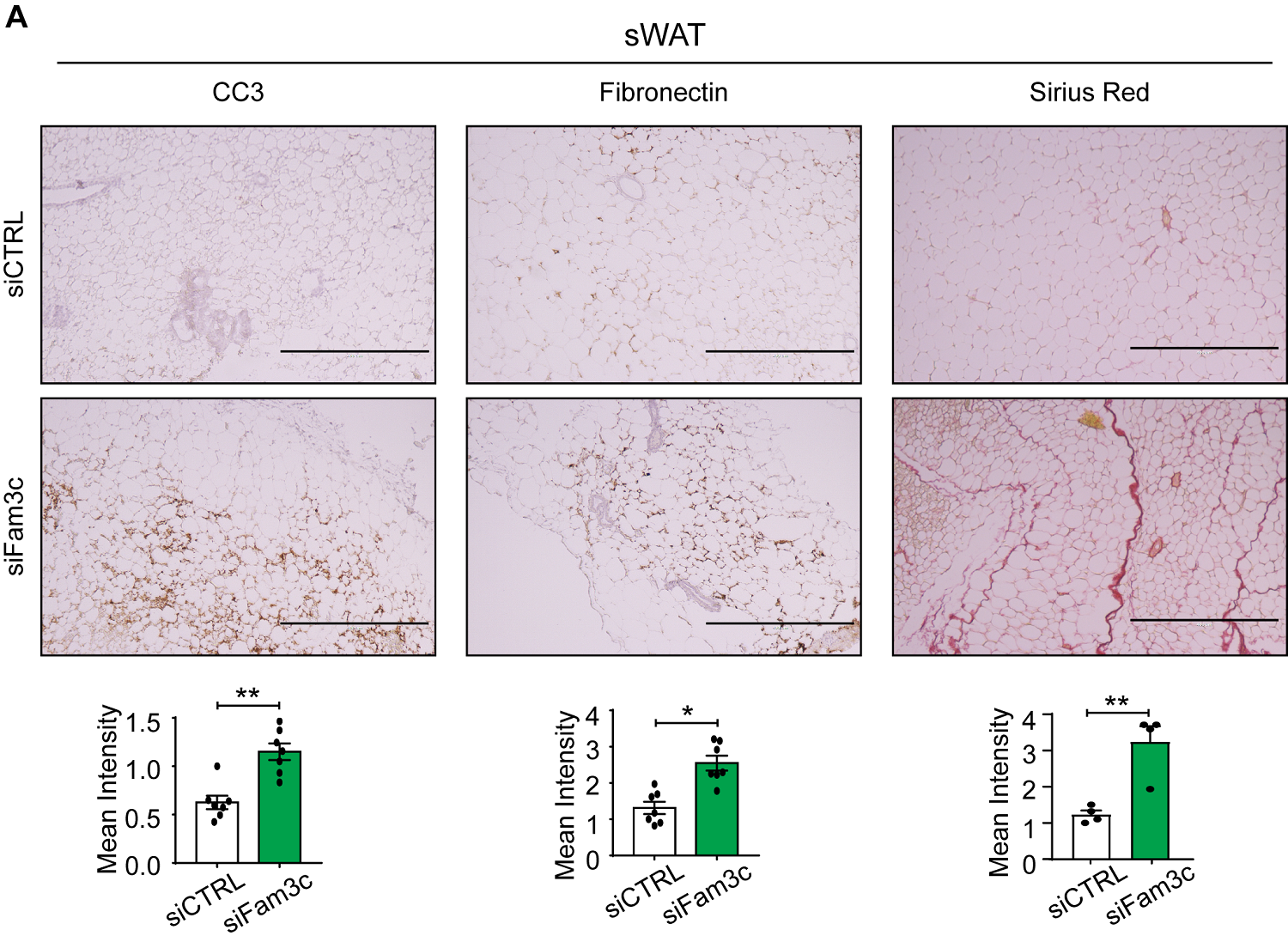
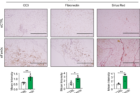
Figure 1. Fam3c knockdown in sWAT of MMTV-PyMT mice increases cell death and fibrosis of CAAs, ultimately inhibiting the growth and metastasis of primary tumors. (left and center) Immunohistochemistry staining images and quantification of CC3 and fibronectin in sWAT from eight-week-old mice. Scale bar: 400 µm. (right) Sirius Red staining images and quantification of sWAT from eight-week-old mice. Scale bar: 400 µm.
The study demonstrates that overexpression of FAM3C in cultured adipocytes significantly reduces cell death in both adipocytes and co-cultured breast cancer cells, while suppressing markers of fibrosis. Conversely, depletion of FAM3C in CAAs leads to adipocyte-mesenchymal transition (AMT) and increased fibrosis within the TME. The research team also discovered that breast cancer cells stimulate the expression of FAM3C in adipocytes through TGF-β signaling, which can be inhibited by a TGF-β-neutralizing antibody.
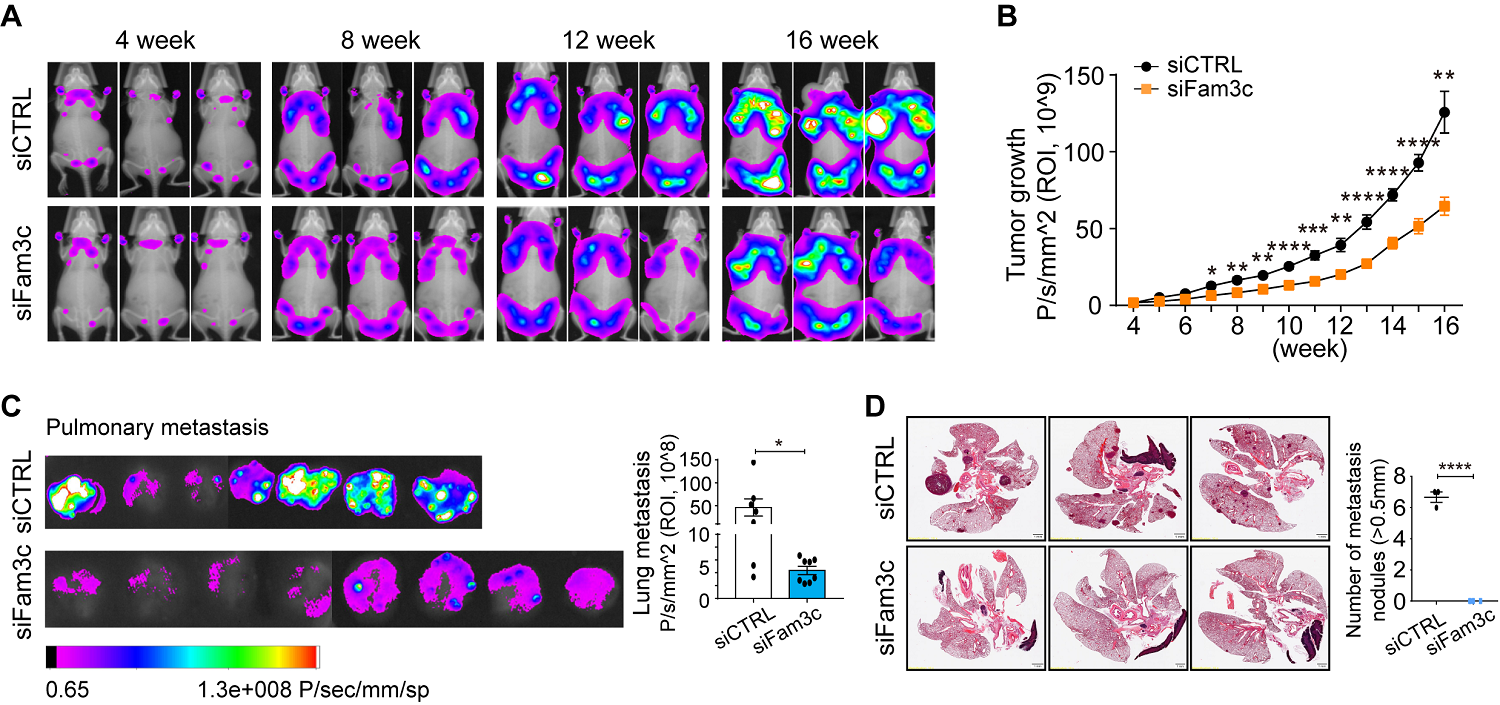
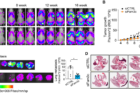
Figure 2. (A) Fluorescence images of whole-body tumor burden. (B) Quantification of the region of interest. (C) Fluorescence images and quantification of lung metastatic burden at 16 weeks. (D) Hematoxylin and eosin staining images of lung metastases and quantification of metastatic nodules with diameters > 0.5 mm. Scale bar: 1 mm.
In a genetically engineered mouse model of breast cancer, early knockdown of FAM3C in CAAs significantly inhibited primary and metastatic tumor growth. Furthermore, elevated levels of circulating FAM3C were observed in patients with metastatic breast cancer compared to those with non-metastatic breast cancer.
“These findings suggest that therapeutic inhibition of FAM3C expression in CAAs during early tumor development could hold promise as a novel approach in the treatment of patients with breast cancer,” said Professor Jiyoung Park. “Understanding the role of cancer-associated adipocytes and their secretory molecules, such as FAM3C, opens up new avenues for the development of early diagnosis markers and targeted treatments for breast cancer.”
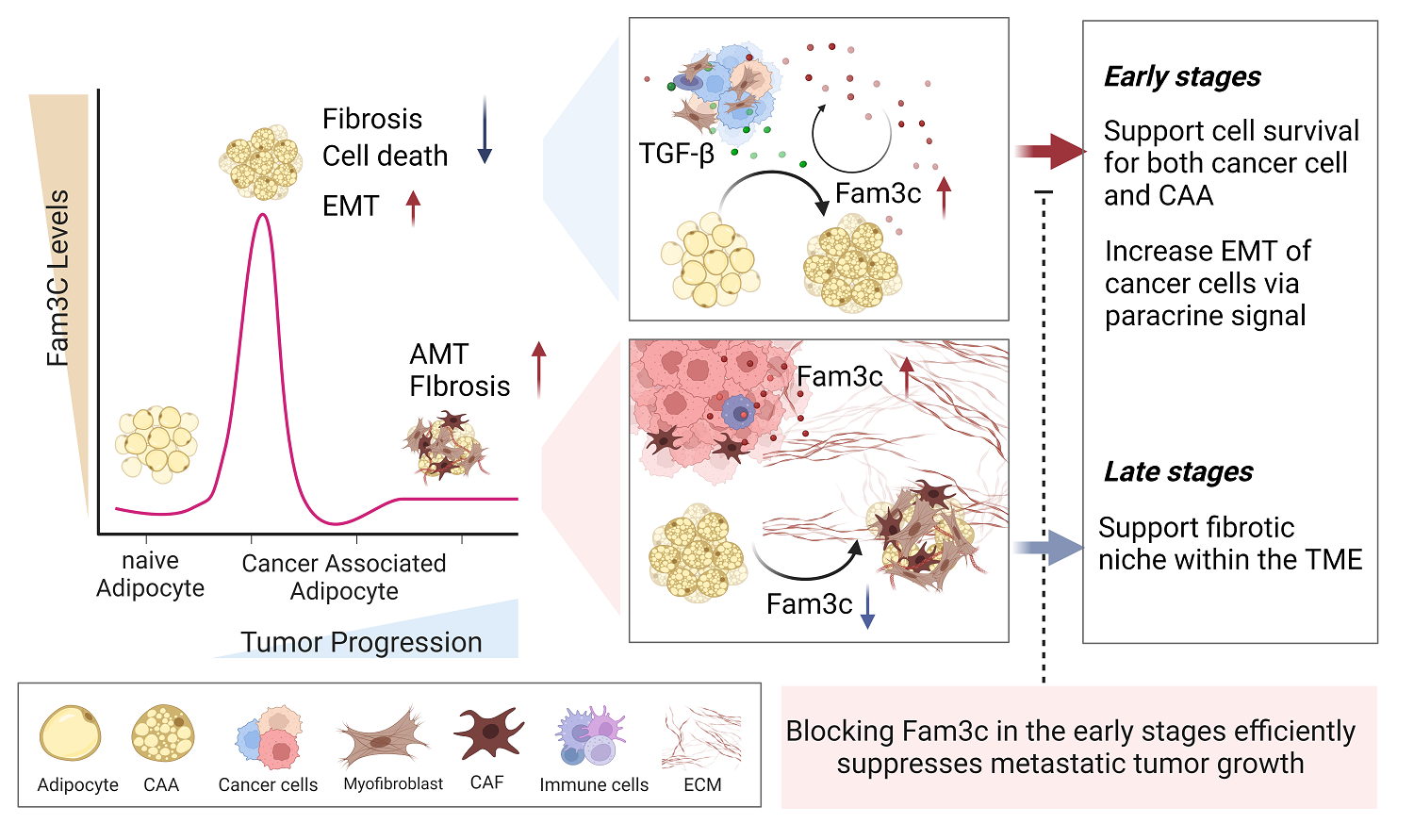
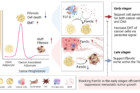
Figure 3. Graphical abstract, showing high FAM3C expression predicts poor prognosis in patients with breast cancer.
The findings of this study have been published in the online version of Cancer Research on December 20, 2023. This research was conducted with the support of the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (MSIT), the Basic Science Research Program, as well as a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea.
Journal Reference
Sahee Kim, Jiyoung Oh, Chanho Park, et al., “FAM3C in cancer-associated adipocytes promotes breast cancer cell survival and metastasis,” Cancer Research, (2023).