A groundbreaking study, led by Professor Sung-Kyun Jung and his research team in the School of Energy and Chemical Engineering at UNIST has unveiled a more stable approach to utilizing all-solid-state batteries (ASSBs), setting a new standard for the development of safe battery systems.
Conventional lithium-ion batteries, powered by organic liquid electrolytes, have long been associated with a high risk of fire and explosion. To mitigate these dangers, the research community has turned its attention to ASSBsc that leverage non-flammable inorganic solid electrolytes. In the pursuit of next-generation solid-state batteries, sulfide solid electrolytes have emerged as promising materials. However, challenges related to thermal instability, stemming from exothermic reactions and explosive decomposition products at the interface between sulfide solid electrolytes and electrode materials, have persisted.
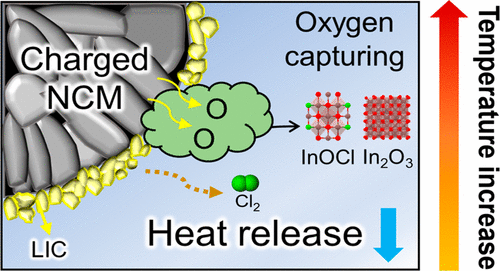
Figure 1. Schematic illustration of thermal decomposition of NCM/LIC composite. As the temperature increases, the NCM/LIC composite shows a delayed phase transition (spinel, 350 °C; rock salt, 500 °C) and mitigated oxygen gas evolution due to the oxygen-capturing effect of LIC.
In a significant breakthrough, the research team explored the use of halide-based solid electrolytes to enhance thermal stability. By replacing sulfide solid electrolytes with halide counterparts, such as Li3InCl6, the team observed improved oxidation stability and reduced oxygen evolution from the cathode.
The study involved the creation of a composite material combining Li3InCl6 (LIC) with a charged cathode material (Li1–xNi0.6Co0.2Mn0.2O2), known as NCM622. The results demonstrated that the halide-based solid electrolyte contributed to delaying the decomposition of NCM622 and suppressing combustible oxygen-gas evolution through an endothermic phase transition process.
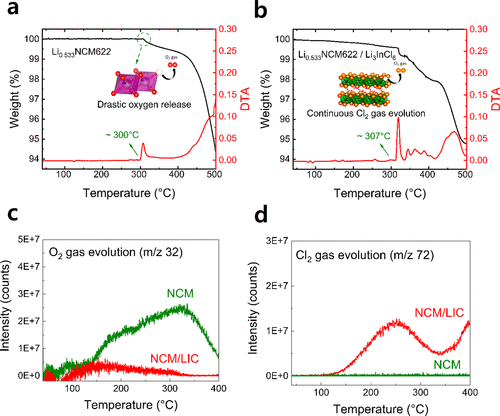
Figure 2. Comparison of thermal decomposition temperature and gas evolution behavior between charged NCM and NCM/LIC composite cathodes.
Notably, the research team observed similar positive outcomes with other halide solid electrolytes, including Li3YCl6 and Li2ZrCl6, across various cathode materials such as LCO. Lead author Sangpyo Lee highlighted the significance of these findings, stating that they offer a novel approach to enhancing the thermal stability of solid-state batteries and provide essential design criteria for safe battery systems in the future.
Professor Jung underscored the pivotal role of the interplay between the cathode and solid electrolyte in governing the thermal stability of ASSBs, emphasizing the potential impact on the design and development of solid electrolytes for secure battery systems.
This research received support from the Korea Research Foundation, the Ministry of Science and ICT through the Civil-Military Technology Cooperation Project, and the Korea Institute of Machinery and Materials. The findings have been published in the online version of ACS Energy Letters on March 4, 2024.
Journal Reference
Sangpyo Lee, Youngkyung Kim, Chanhyun Park, et al., “Interplay of Cathode–Halide Solid Electrolyte in Enhancing Thermal Stability of Charged Cathode Material in All-Solid-State Batteries,” ACS Energy Lett., (2024).