A groundbreaking study led by Professor Jae-Ick Kim from the Department of Biological Sciences at UNIST has uncovered a novel mechanism for controlling dopamine secretion. The research, published in the prestigious journal Experimental & Molecular Medicine, highlights the role of phospholipase Cγ1 (PLCγ1), a crucial protein involved in intracellular signaling, in regulating dopamine release.
Dopamine, a neurotransmitter essential for voluntary movement, reward learning, and motivation, is closely tied to various psychological and neurodegenerative disorders. Understanding the intricate signaling mechanisms that modulate dopamine neurons is vital for the development of effective therapeutic strategies against dopamine-related diseases.
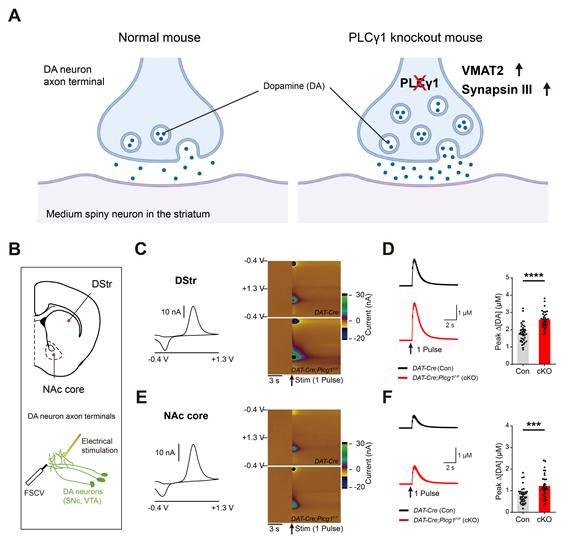
Figure 1. Genetic deletion of PLCγ1 enhances the release of dopamine at dopaminergic terminals.
Previous studies had suggested that PLCγ1 may be implicated in the development of dopaminergic neurons. However, the precise physiological function of PLCγ1 remained elusive. To address this knowledge gap, Professor Kim’s team conducted an in-depth investigation into the role of PLCγ1 in regulating dopaminergic function in vivo.
The study utilized cell type-specific deletion of PLCγ1 in dopamine neurons to evaluate its impact on dopamine release. Surprisingly, the deletion of PLCγ1 did not adversely affect the development and cellular morphology of midbrain dopamine neurons. Instead, it led to a notable increase in dopamine release from dopaminergic axon terminals in the striatum.
The enhanced dopamine release observed in the study was accompanied by an upregulation of vesicular monoamine transporter 2 (VMAT2) at dopaminergic axon terminals. VMAT2 is responsible for transporting dopamine into synaptic vesicles. Additionally, the knockout of PLCγ1 in dopamine neurons resulted in heightened expression and colocalization of synapsin III, a protein that controls the trafficking of synaptic vesicles.
Furthermore, the study demonstrated that the knockdown of both VMAT2 and synapsin III in dopamine neurons significantly attenuated dopamine release. However, this attenuation was less severe in PLCγ1 knockout mice, suggesting that PLCγ1 critically modulates dopamine release at axon terminals by directly or indirectly interacting with synaptic machinery.
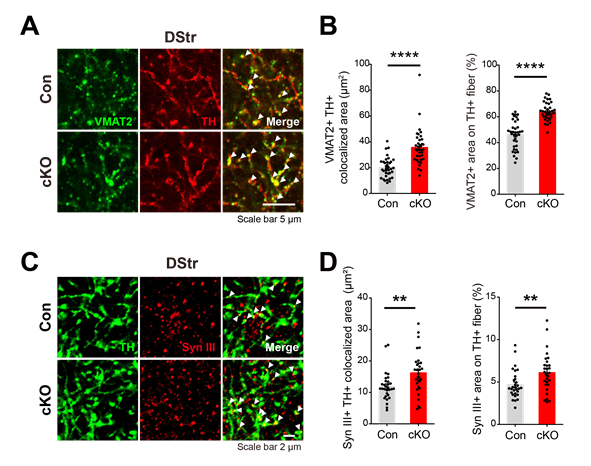
Figure 2. Altered synapsin III expression might underlie the increased localization of VMAT2 in the dopaminergic axons of PLCγ1 cKO mice. (A) Representative confocal images of VMAT2 expression in striatal dopaminergic axons (white arrowhead: colocalized area). (B) VMAT2 and TH colocalized area and the percentage of VMAT-positive area on TH-positive axons. (C) Representative confocal images showing the expression of synapsin III in striatal dopaminergic axons (white arrowhead: colocalized area). (D) Synapsin III and TH colocalized area in the DStr and the percentage of synapsin III-positive area on TH-positive axons in the DStr.
Professor Kim explained, “Our study provides novel insights into the signaling mechanisms within dopamine neurons, which were previously challenging to decipher. By identifying the role of dopamine neuron-specific PLCγ1, we have paved the way for potential advancements in the treatment of dopamine-related brain diseases.”
Hye Yun Kim, the first author of the study, expressed optimism regarding the future impact of their findings. “By elucidating the PLCγ1-mediated signaling pathways involved in the regulation of dopamine secretion, we hope to contribute to the development of innovative treatments for dopamine-related brain diseases.”
The research was conducted in collaboration with the Korea Brain Research Institute (KBRI) and received support from the Mid-Career Researcher Program and the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (MSIT).
Journal Reference
Hye Yun Kim, Jieun Lee, Hyun-Jin Kim, et al., “PLCγ1 in dopamine neurons critically regulates striatal dopamine release via VMAT2 and synapsin III,” EMM, (2023).