The quality and restfulness of your sleep are heavily influenced by environmental conditions, such as noise, light, humidity, and nutrient intakes. Besides, the temperature of our surrounding environment is another important factor that influences the quantity and quality of sleep. A recent study, which explains the relationship between the ambient temperature and quality of sleep, has attracted attention from academia.
A research team, led by Professor Chunghun Lim in the School of Life Sciences at UNIST has succeeded in unveiling the principle behind the changing sleep patterns, according to the ambient temperature, through the use of Drosophila as a model of sleep. In other words, the neuronal junction (synapses) between sleep-regulating neurons that sends and receives signals, using GABA (Gamma-Amino Butyric Acid), the major inhibitory neurotransmitter in the brain, disappears when the temperature rises, and thus changes the sleep pattern.
During extreme heatwaves, people tend to experience sleepiness throughout the day and have difficulty falling asleep at night. Drosophila, likewise, is less active during the day in hot environments and does not sleep well at night. To find the neurophysiological principle of this phenomenon, the research team cultured the genetically engineered fruit flies under temperature, similar to hot summer days, and observed their sleep patterns.
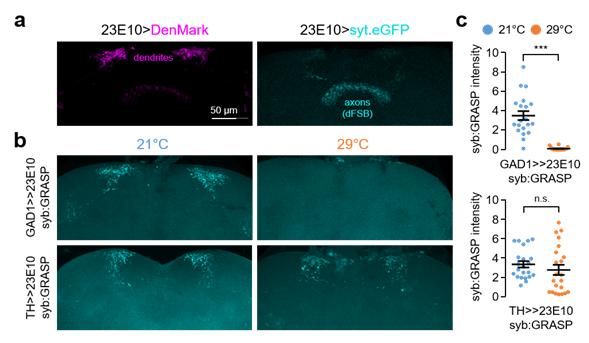
Figure 1: GABAergic synapses, but not dopaminergic synapses, onto dFSB neurons display temperature-sensitive activity.
The fruit flies used in the experiment have the mutation in the ‘Shaker (Sh)’ gene. The protein produced by this gene creates a pathway through which potassium ions (K⁺) pass in the brain. If this protein is deficient, it activates nerve cells excessively to suppress sleep. After all, mutant fruit flies sleep less than other fruit flies.
However, even when the same type of fruit fly was cultured in hot environments, sleep suppression did not appear. The study noted that this phenomenon is due to the disappearance of the link between dFSB (dorsal Fan-Shaped Body neuron) and the inhibitory neurotransmitter GABA.
In the study, the research team demonstrated that Shaker (Sh)-expressing GABAergic neurons, projecting onto dorsal fan-shaped body (dFSB) regulate temperature-adaptive sleep behaviors in Drosophila. Loss of Sh function suppressed sleep at low temperature whereas light and high temperature cooperatively gated Sh effects on sleep. Sh depletion in GABAergic neurons partially phenocopied Sh mutants.
“Our study establishes a genetic pathway that constitutes temperature-sensitive GABA transmission to the sleep-promoting neural locus and generates neural plasticity underpinning the adaptive organization of sleep architecture,” says Professor Lim. “Knowing the changes in sleep patterns, caused by springtime lethargy and heatwaves, will help us better resolve—or help treat—sleep disorders.”
The findings of this research have been published in Communications Biology on April 15, 2020. This work has been supported by grants from the Suh Kyungbae Foundation, as well as the Advanced Research Center Program from the National Research Foundation (NRF) funded by the Ministry of Science and ICT (MSIT). It has been also supported by the Global Ph.D. Fellowship project.
Journal Reference
Ji-hyung Kim, Yoonhee Ki, Hoyeon Lee, et al., “The voltage-gated potassium channel Shaker promotes sleep via thermosensitive GABA transmission,” Communications Biology, (2020).