A research team, led by Professor Dong Woog Lee from the Department of Chemistry at UNIST, in collaboration with Professor Byeong-Su Kim from the Department of Chemistry at Yonsei University, has discovered that the synergistic anion–π interactions serves as a key principle in enhancing the cohesion of synthetic polymers.
In this groundbreaking study, the researchers developed an epoxy monomer-based polymer that mimics the structural features of mussel foot proteins and experimentally demonstrated that anion–π interactions are pivotal in strengthening polymer cohesion.
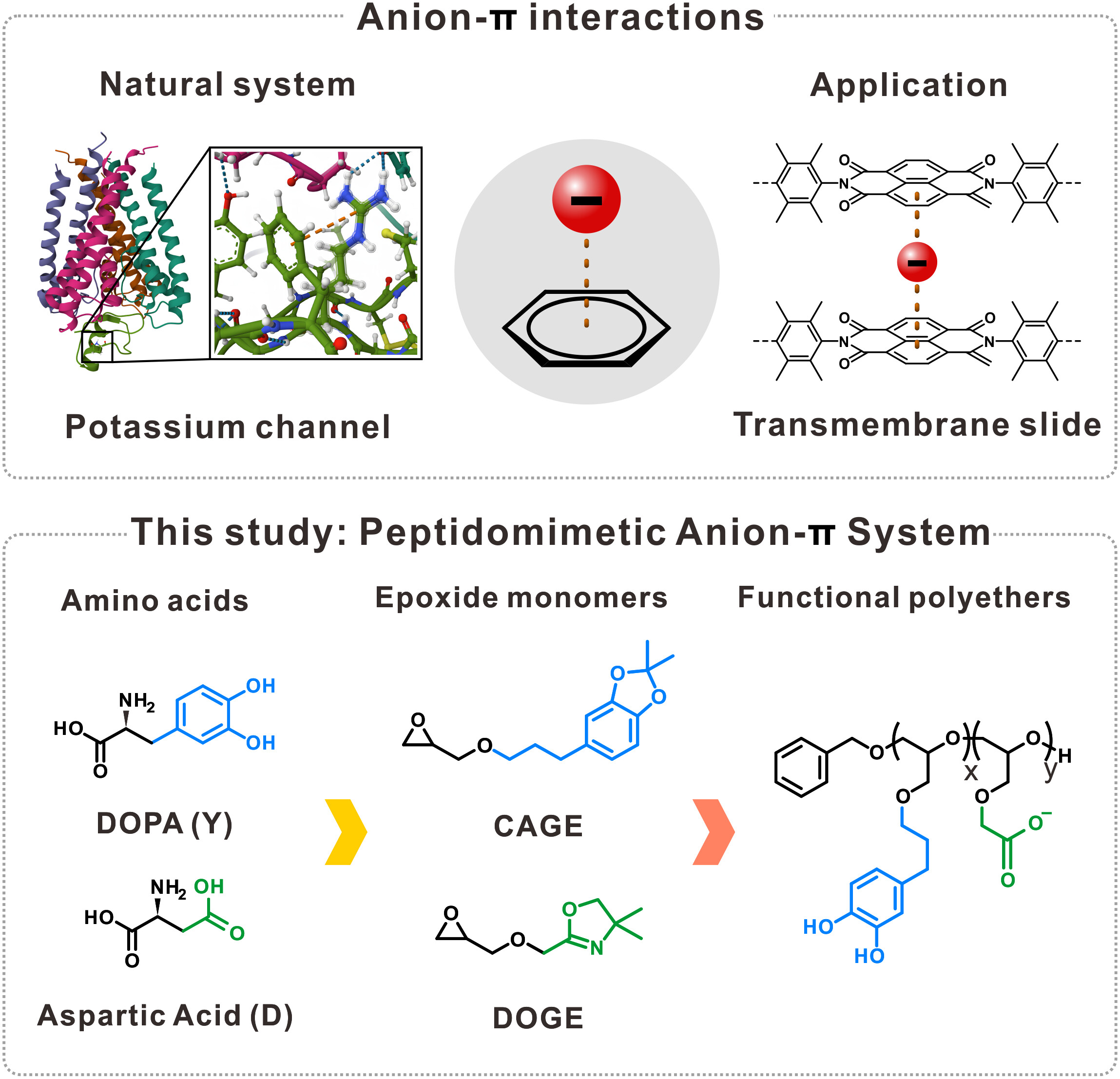 Figure 1. (Top) Representative examples of anion–π interactions exploited in natural and synthetic systems and (Bottom) the peptidomimetic system explored in this study. l Image Credit: Department of Chemistry, Yonsei University
Figure 1. (Top) Representative examples of anion–π interactions exploited in natural and synthetic systems and (Bottom) the peptidomimetic system explored in this study. l Image Credit: Department of Chemistry, Yonsei University
Anion–π interactions are non-covalent bonds formed between negatively charged molecules (anions) and the π electron systems of aromatic rings. While these interactions are known to play critical roles in biological processes such as enzyme catalysis and ion transport, research exploring their application in synthetic polymers remains scarce.
Inspired by mussels, which exhibit remarkable adhesive properties in natural environments, the research team focused on the plantar proteins of these organisms. Through an analysis of the key components contributing to their strong binding capabilities, the scientists found that the structural characteristics of 3,4-dihydroxyphenylalanine (DOPA) and aspartic acid are particularly significant.
To advance their findings, the research team designed functional monomers that replicate these structural features, leading to the synthesis of a novel polymer. This work proposes a new design methodology for polymers, taking into account the complex intermolecular interactions present in biological systems.
Specifically, the monomer that emulates the DOPA structure provides the π-electronic field of the aromatic ring, while the monomer representing aspartic acid introduces the anion necessary for anion–π interactions within the polymer framework. Furthermore, the team employed a surface force apparatus (SFA) to quantitatively analyze the cohesiveness of the polymer under various conditions.
The team compared the cohesion of the polymer in neutral environments, where its functional groups are ionized, against acidic conditions, where they remain non-ionized. Their findings revealed that in neutral environments, anion–π interactions serve as the principal binding force, significantly enhancing polymer cohesion. In contrast, under acidic conditions, hydrogen bonding dominates, resulting in comparatively weaker cohesion.
This study marks the first experimental evidence highlighting the decisive role of anion–π interactions in reinforcing cohesion among synthetic polymers. The implications of these findings open avenues for innovative polymer design strategies applicable in diverse fields, including adhesives, self-assembly systems, catalysts, and drug delivery.
This research was supported by the National Research Foundation of Korea (NRF) and the Ministry of Science and ICT (MSIT). The findings of this study were published in the online version of Proceedings of the National Academy of Sciences (PNAS) on February 6, 2025.
Journal Reference
Seunghyun Lee, Aram Shin, Jinwoo Park, et al., “Synergistic anion–π interactions in peptidomimetic polyethers,” PNAS, (2025).












