A research team, led by Professor Ja-Young Ryu from the Department of Chemistry at UNIST has introduced an innovative technology that selectively targets and destroys cancer cells by utilizing a protein-mimicking polymer that is generated exclusively within these cells.
This advanced method leverages enzymes that are overexpressed in cancer cells, leading to the creation of a polymer that is significantly safer compared to conventional chemotherapy, as it does not cause toxicity to normal healthy cells
Existing polymerization techniques have faced challenges in reliably distinguishing between cancerous and healthy cells. Nonetheless, the team has developed a polymerization strategy that selectively affects cancer cells. In this context, polymerization involves the ongoing combination of small molecules into larger, more complex structures.
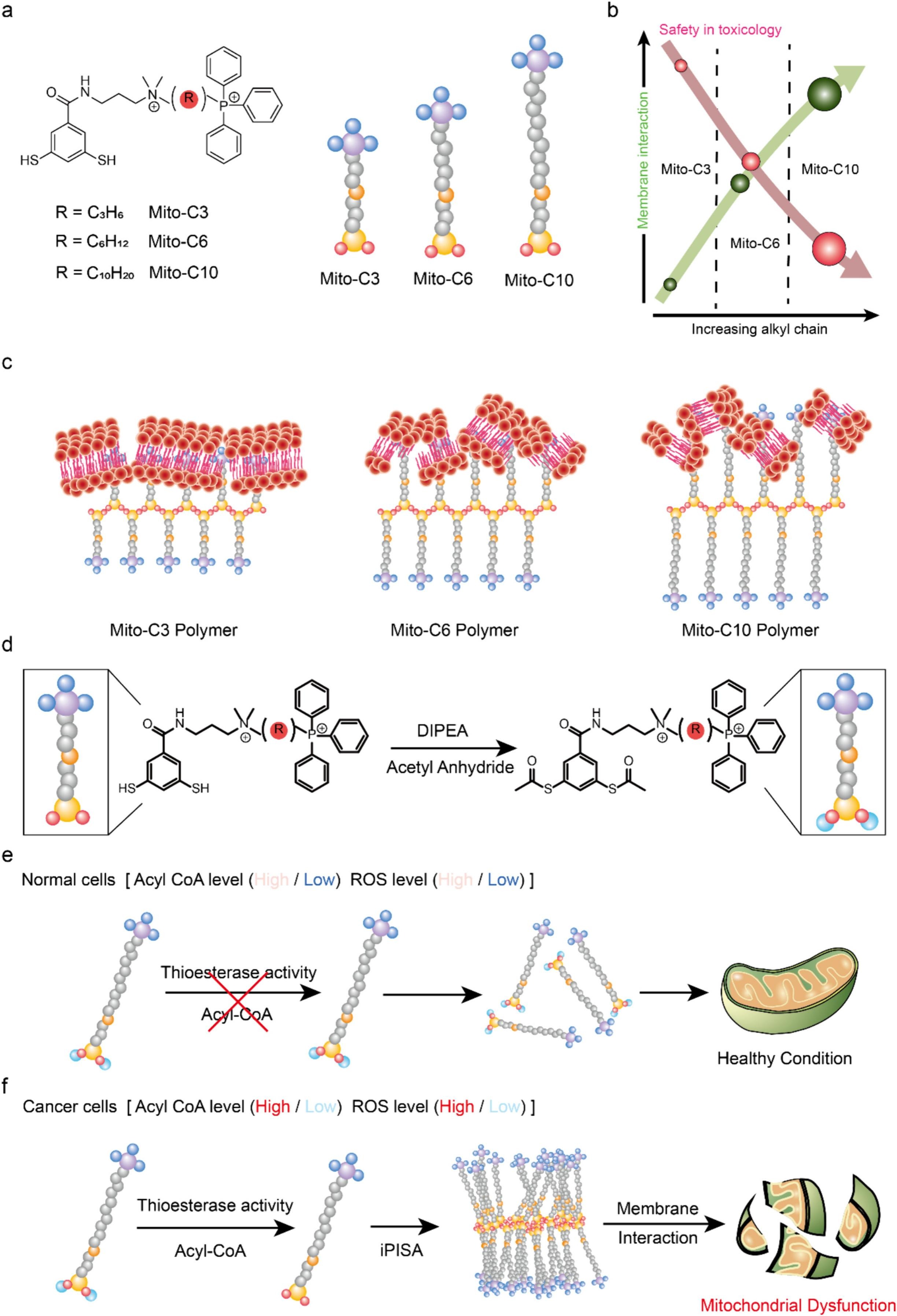 Figure 1. (a) Chemical structure of mitochondria targeting monomers with increasing alkyl chain length. (b) Schematic illustration showing the relationship between membrane interaction and biosafety of polymeric structures resulting from polymerization-induced self-assembly of Mito-C3, Mito-C6, and Mito-C10. (c) Cartoon image demonstrating the extent of membrane disruption by polymeric structures. Mito-C10, Mito-C6 and Mito-C3 polymers destroy mitochondrial membranes in order. (d) Chemical reactions for acetylation of thiol groups to prevent unexpected disulfide formation. (e,f) The thioesterase-mediated polymerization is triggered specifically by a cancerous environment, in which ACOT and ROS were overexpressed, thus selective construction of the polymeric structures. The resulting structures destroyed mitochondria.
Figure 1. (a) Chemical structure of mitochondria targeting monomers with increasing alkyl chain length. (b) Schematic illustration showing the relationship between membrane interaction and biosafety of polymeric structures resulting from polymerization-induced self-assembly of Mito-C3, Mito-C6, and Mito-C10. (c) Cartoon image demonstrating the extent of membrane disruption by polymeric structures. Mito-C10, Mito-C6 and Mito-C3 polymers destroy mitochondrial membranes in order. (d) Chemical reactions for acetylation of thiol groups to prevent unexpected disulfide formation. (e,f) The thioesterase-mediated polymerization is triggered specifically by a cancerous environment, in which ACOT and ROS were overexpressed, thus selective construction of the polymeric structures. The resulting structures destroyed mitochondria.
The researchers created a distinctive monomer that responds to a specific enzyme. When activated by this enzyme, the monomer undergoes polymerization, resulting in a structure that disrupts the mitochondria of cancer cells, leading to oxidative stress that inhibits tumor growth.
Importantly, cancer cells show an elevated expression of thioesterases compared to their normal counterparts. This enzyme-triggered polymerization occurs exclusively in cancer cells, ensuring targeted elimination without the potential for drug resistance development.
Professor Ryu stated, “We have developed a system that can regulate the fate of cells by enabling polymer formation specifically within them.” He further added, “We anticipate that this new technology will lead to more effective chemotherapy treatments.”
The findings of this research were published in the Journal of Controlled Release on July 16, 2024. The study was conducted with support from the Ministry of Science and ICT (MSIT) and the National Research Foundation of Korea (NRF).
Journal Reference
Sangpil Kim, Yeji Lee, Min-Seok Seu, et al., “Enzyme-instructed intramitochondrial polymerization for enhanced anticancer treatment without the development of drug-resistance,” JCR, (2024).












