A research team, affiliated with UNIST has introduced a novel approach that targets and destroys proteins used by cancer cells to escape immune detection, potentially opening new avenues for effective cancer therapies.
Led by Professor Ja-Hyoung Ryu in the Department of Chemistry at UNIST, the team has pioneered a groundbreaking composite assembly technology capable of degrading immune-evasion proteins on cancer cells. This innovative method involves encapsulating these proteins within a complex that directs them to lysosomes—cellular structures dedicated to breaking down proteins. By creating an environment conducive to immune recognition and attack, this technology promises to enhance the efficacy of immunotherapies.
Cancer cells often overexpress the protein PD-L1 on their surfaces, which transmits inhibitory signals to immune cells, effectively ‘turning off’ immune responses and allowing tumors to proliferate unchecked. Overcoming this immune suppression is critical for advancing cancer treatments.
The research team developed a technology based on acetazolamide that selectively degrades PD-L1 on cancer cells. Acetazolamide binds specifically to CAIX, an enzyme predominantly expressed on the surface of cancer cells, forming nano-sized protein complexes. These complexes simultaneously carry immune-evasion proteins like PD-L1 into the cell’s interior. Once inside, the nanocomplexes are recognized as abnormal proteins and are degraded within lysosomes—the cell’s ‘waste disposal’ organelles. Since CAIX is scarcely present in normal cells, this reaction is highly selective for cancer cells, minimizing potential side effects.
In animal experiments involving mice, administration of this complex resulted in more than a 50% reduction in tumor size and a significant decrease in PD-L1 levels on cancer cells. These findings demonstrate the potential of this technology to restore immune system recognition and facilitate tumor elimination.
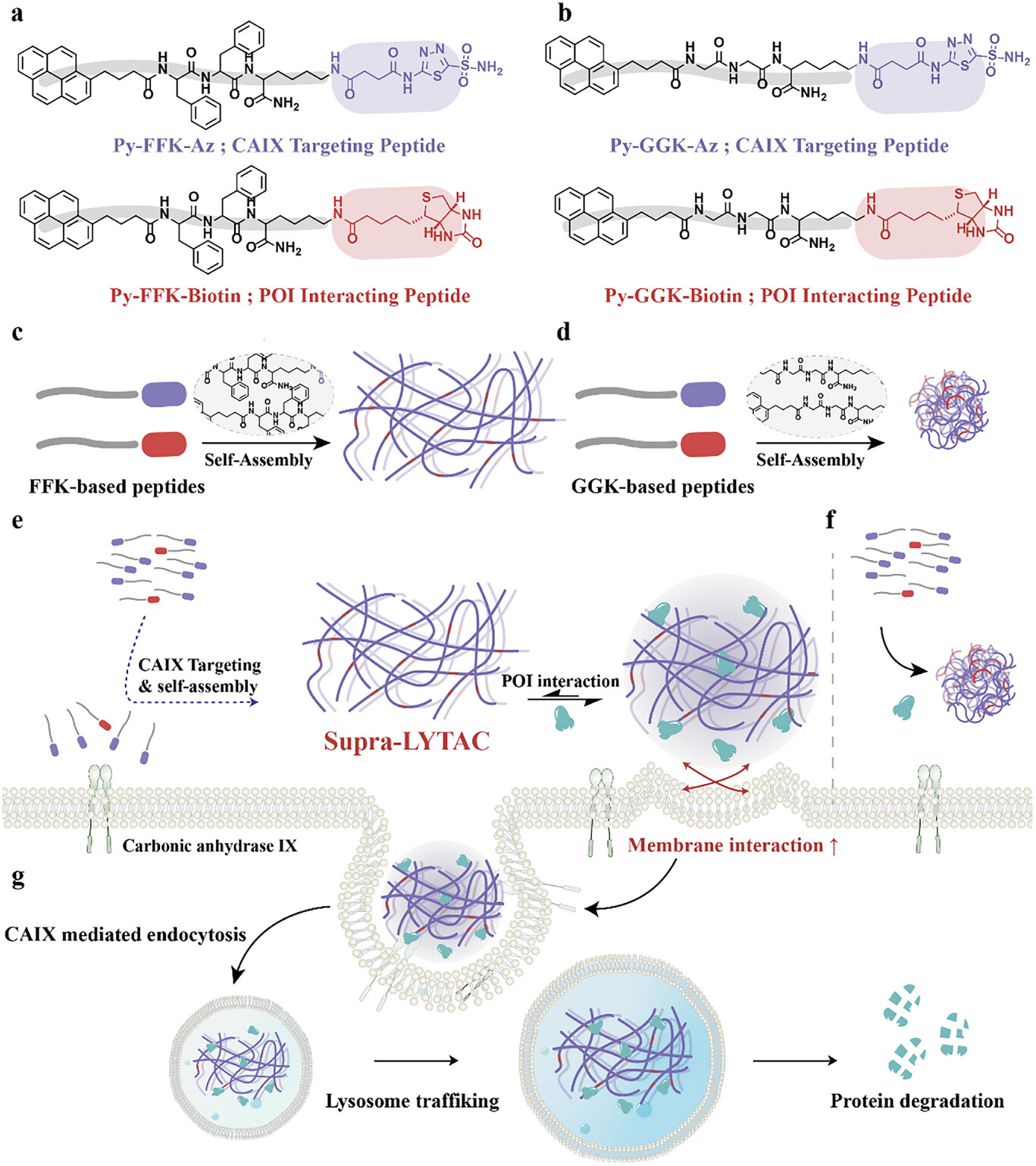 Scheme 1. a,b) Monomer design for Supra-LYTAC; c,d) Illustration demonstrating co-assembly to yield nanofiber and nanosphere. The nanofiber structure could be constructed in FFK-based peptides due to the high self-assembly propensity, while GGK-based peptides generate nanosphere structure; e) Supra-LYTAC constructed in the cancerous membrane by targeting CAIX. It can further interact with POI, as followed by internalization into lysosome via CAIX-induced endocytosis; f) Nanosphere showing weak ability for the internalization into lysosome due to low self-assembly propensity; g) POI internalizing into lysosome with Supra-LYTAC could be degraded in the lysosome.
Scheme 1. a,b) Monomer design for Supra-LYTAC; c,d) Illustration demonstrating co-assembly to yield nanofiber and nanosphere. The nanofiber structure could be constructed in FFK-based peptides due to the high self-assembly propensity, while GGK-based peptides generate nanosphere structure; e) Supra-LYTAC constructed in the cancerous membrane by targeting CAIX. It can further interact with POI, as followed by internalization into lysosome via CAIX-induced endocytosis; f) Nanosphere showing weak ability for the internalization into lysosome due to low self-assembly propensity; g) POI internalizing into lysosome with Supra-LYTAC could be degraded in the lysosome.
Dohyun Kim, the first author of the study, stated, “We aim to further explore additional pathways through which the immune system can directly target and eliminate cancer.”
While existing protein-degrading technologies such as PROTACs and LYTACs have shown promise, they often face limitations related to molecule size and cellular entry due to their complex structures. To overcome these hurdles, the research introduces a new approach where the molecules self-assemble within the body, enabling more efficient and targeted protein degradation.
Professor Ryu commented, “This represents a new form of targeted protein degradation technology that surpasses the limitations of conventional polymer-based chimeras. It has significant potential for combination therapy with immunotherapies and for treating various refractory solid tumors.”
The study was published in Advanced Science on April 3 and was supported by the Ministry of Science and ICT and the National Research Foundation of Korea.
Journal Reference
Dohyun Kim, Gyeongseok Yang, Chaelyeong Lim, et al., “Cancer Specific CAIX-Targeting Supramolecular Lysosome-Targeting Chimeras (Supra-LYTAC) for Targeted Protein Degradation,” Adv. Sci., (2025).