A research team, affiliated with UNIST has unveiled a novel extracorporeal blood purification technology that captures and removes bacteria from the bloodstream by leveraging sticky, clot-like surfaces. This breakthrough could pave the way for new treatments against deadly systemic infections, including sepsis, even those caused by antibiotic-resistant bacteria.
Led by Professor Joo H. Kang from the Department of Biomedical Engineering at UNIST, the research team announced the development of an innovative extracorporeal bacterial purification device that utilizes artificial blood clots. Similar to dialysis, the technique involves extracting infected blood outside the body, adsorbing bacteria onto artificial thrombi, and then returning the purified blood to the patient.
The newly developed extracorporeal blood purification device (eCDTF) features a spiral structure inserted into the central tube. Inside this spiral, artificial blood clots are embedded, which attract and trap bacteria flowing through the tube. Composed solely of plasma proteins without any cellular components like white blood cells, these artificial thrombi facilitate effective bacterial adhesion to the device surface.
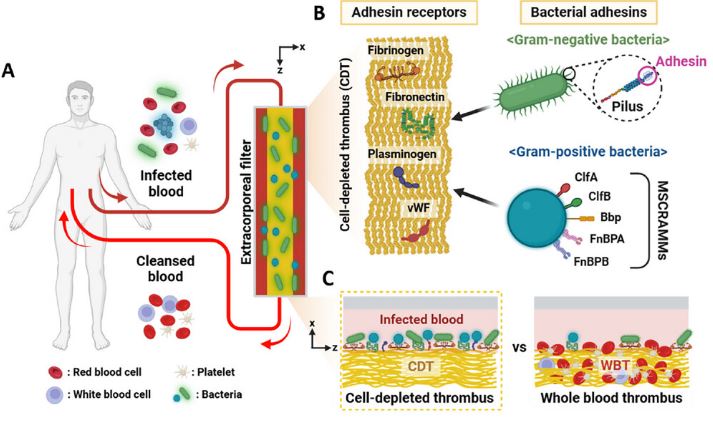 Figure 1. The universal bacterial-capturing capability of adhesin receptor proteins on the cell-depleted thrombus (CDT). A) The extracorporeal blood-cleansing system through hemoadsorption onto the functional CDT surface. B) The CDT comprises the adhesin receptor proteins, including fibrinogen, fibronectin, plasminogen, and von Willebrand factor (vWF), which bind bacterial pili of Gram-negative bacteria and microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) of Gram-positive bacteria.
Figure 1. The universal bacterial-capturing capability of adhesin receptor proteins on the cell-depleted thrombus (CDT). A) The extracorporeal blood-cleansing system through hemoadsorption onto the functional CDT surface. B) The CDT comprises the adhesin receptor proteins, including fibrinogen, fibronectin, plasminogen, and von Willebrand factor (vWF), which bind bacterial pili of Gram-negative bacteria and microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) of Gram-positive bacteria.
The eCDTF demonstrated the ability to effectively remove over 90% of major pathogenic bacteria species, including both Gram-positive and Gram-negative strains such as Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli), as well as antibiotic-resistant strains and bacteria derived from human fecal matter.
Preclinical experiments further validated its efficacy. In tests involving rats infected with methicillin-resistant Staphylococcus aureus (MRSA), just three hours of extracorporeal circulation significantly reduced blood bacterial counts and inflammatory markers. Major organs such as the liver and spleen, which are common sites for bacterial invasion, also showed substantial decreases in bacterial load. Notably, while all untreated control rats succumbed within seven days, those treated once with the device achieved a survival rate of approximately 33%, and those treated twice achieved 100%.
The research team developed this technology based on insights into blood flow dynamics. In circulating blood, flexible red blood cells tend to migrate toward the center of flow, while stiffer platelets are pushed toward the vessel walls—a phenomenon known as margination. The team hypothesized that bacteria, which are similarly small and rigid like platelets, could be directed and captured through this mechanism. The device’s structure and blood flow parameters were optimized to maximize bacterial margination and removal.
Professor Kang stated, “This technology can directly eliminate a wide range of pathogenic bacteria without the use of antibiotics, potentially transforming the treatment of bloodstream infections such as bacteremia and sepsis.” He added, “If not treated promptly, bloodstream infections can escalate into sepsis, causing widespread inflammation and organ failure. Our device offers a promising new approach to intervene early and effectively.”
He further added, “This development allows us to scientifically explain previously unexplained bacterial removal phenomena observed with existing devices, further enhancing the clinical potential of this technology.”
This research was supported by various organizations, including the Ministry of Science and ICT (MSIT), the National Research Foundation of Korea (NRF), and the Ministry of Health and Welfare (MOHW), and the Ministry of Trade Industry & Energy (MOTIE). Their findings have been published online in the international journal, Advanced Science on April 26, 2025.
Journal Reference
Bong Hwan Jang, Su Hyun Jung, Seyong Kwon, et al., “Red Blood Cell-Induced Bacterial Margination Improves Microbial Hemoadsorption on Engineered Cell-Depleted Thrombi, Restoring Severe Bacteremia in Rats,” Adv. Sci., (2025).