A research team, led by Professor Dong Woog Lee in the School of Energy and Chemical Engineering at UNIST has developed a novel semi-crystalline polymer binder that offers exceptional electrical conductivity and underwater adhesion, promising to significantly improve the performance and lifespan of seawater-based energy storage systems.
Batteries consist of complex structures composed of multiple materials, and the binder that holds these materials together plays a crucial role in determining overall performance. Specifically, seawater batteries must operate reliably in aquatic environments over extended periods. Therefore, developing a binder that combines strong underwater adhesion with efficient electrical conduction is essential.
The team engineered a semi-crystalline polymer binder characterized by a unique blend of amorphous and crystalline regions within a single material. The crystalline domains, with their regular molecular arrangements, facilitate rapid electron transport by providing efficient conduction pathways, while the amorphous regions allow flexible movement of polymer chains, enhancing adhesion to surfaces and catalysts.
Application of this innovative binder resulted in a tripling of battery lifespan compared to conventional PVDF binders. While batteries with PVDF experienced significant performance degradation within 120 hours of operation, those utilizing the new binder demonstrated stable performance for over 400 hours and maintained long-term operation for up to 1,200 hours.
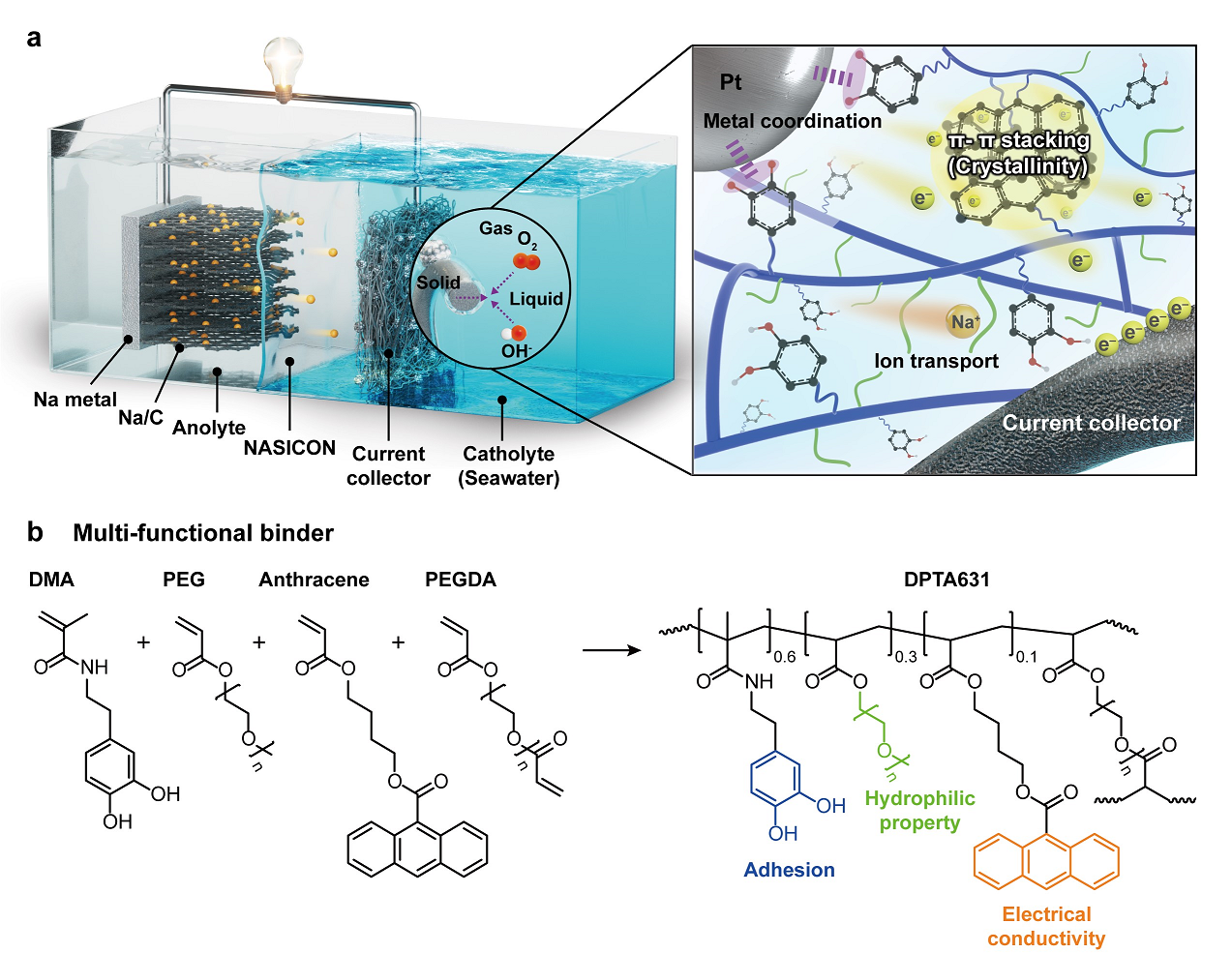 Figure 1. Schematic image, illustrating the aqueous sodium–air batteries. (a) air electrode assembled with a polymer binder, Pt catalyst, and carbon current collector in aqueous media, and (b) molecular design of a multi-functional binder with underwater adhesion and electrical conductivity.
Figure 1. Schematic image, illustrating the aqueous sodium–air batteries. (a) air electrode assembled with a polymer binder, Pt catalyst, and carbon current collector in aqueous media, and (b) molecular design of a multi-functional binder with underwater adhesion and electrical conductivity.
Furthermore, the overvoltage—a factor affecting reaction efficiency—was reduced by up to 66%, enabling the batteries to operate with less energy input. The energy output during discharge increased by 26%, and maximum power output nearly doubled, increasing by 96%.
The research team analyzed the crystalline structure, electrical conductivity, and adhesion properties of the binder to understand the mechanisms behind these performance enhancements. Results showed that the crystalline regions act as rapid electron highways, providing direct pathways for electron flow, while the amorphous regions facilitate flexible interactions. The excellent adhesion was attributed to metal coordination bonds formed between the binder’s amorphous regions and metal catalysts.
Importantly, the developed binder is free of fluorinated compounds, making it compliant with European Union regulations targeting PFAS (per- and polyfluoroalkyl substances). This positions the material as a promising candidate for environmentally friendly electric vehicle batteries, as the EU plans to phase out the use of PVDF and other PFAS-based materials due to their environmental persistence and health concerns.
Jeonguk Hwang, the first author of the study, stated, “This research demonstrates that by designing semi-crystalline polymers, we can overcome the limitations of existing binders and significantly enhance seawater battery performance. We anticipate broad applications across various electronic materials, aqueous metal batteries, and energy storage systems.”
This study was conducted in collaboration with Professors Seok Ju Kang, Hyun-Wook Lee, Youngsik Kim, and Hyunhyub Ko from the School of Energy and Chemical Engineering at UNIST, as well as Professor Tae Joo Shin from the Graduate School of Semiconductor Materials and Devices Engineering at UNIST. The research was supported by the Mid-Career Researcher Program, Creative Challenging Research Program, and Nano & Material Technology Development Program, funded by the National Research Foundation of Korea (NRF) and the Ministry pf Science and ICT (MSIT).
The findings were published in the prestigious journal, Energy & Environmental Science (IF 32.4) on March 31, 2025.
Journal Reference
Jeonguk Hwang, Min Hoon Myung, Jee Ho Ha, et al., “A semi-crystalline polymer binder with enhanced electrical conductivity and strong underwater adhesion in aqueous sodium–air batteries,” Energy Environ. Sci., (2025).












