Recent research has confirmed that toxic gases can be effectively purified at specific sites of enzymes.
Professor Yong Hwan Kim and his research team from the Graduate School of Carbon Neutrality and the School of Energy and Chemical Engineering at UNIST, in collaboration with Professor Jungki Ryu from the School of Energy and Chemical Engineering at UNIST and Professor Hyung Ho Lee from Seoul National University, has shed light on the capabilities of Carbon Monoxide Dehydrogenase (CODH) for the first time.

Figure 1. Proposed model of EV interaction with F41 in the CO oxidation of CODH.
The study revealed that CODH has the ability to completely purify up to 100% of harmful gases found in industrial waste gas, thus contributing to a cleaner and safer environment. Although CODH is primarily known for converting carbon monoxide into industrial waste, the specific electron transport site of this enzyme has remained a mystery until now.
Through their research, the team identified a specific electron transfer action site of CODH. This site facilitates an oxidation reaction that results in the loss of electrons. The electrons produced during this reaction are then transferred through the electron transport system, allowing for the purification of harmful gases like carbon monoxide.
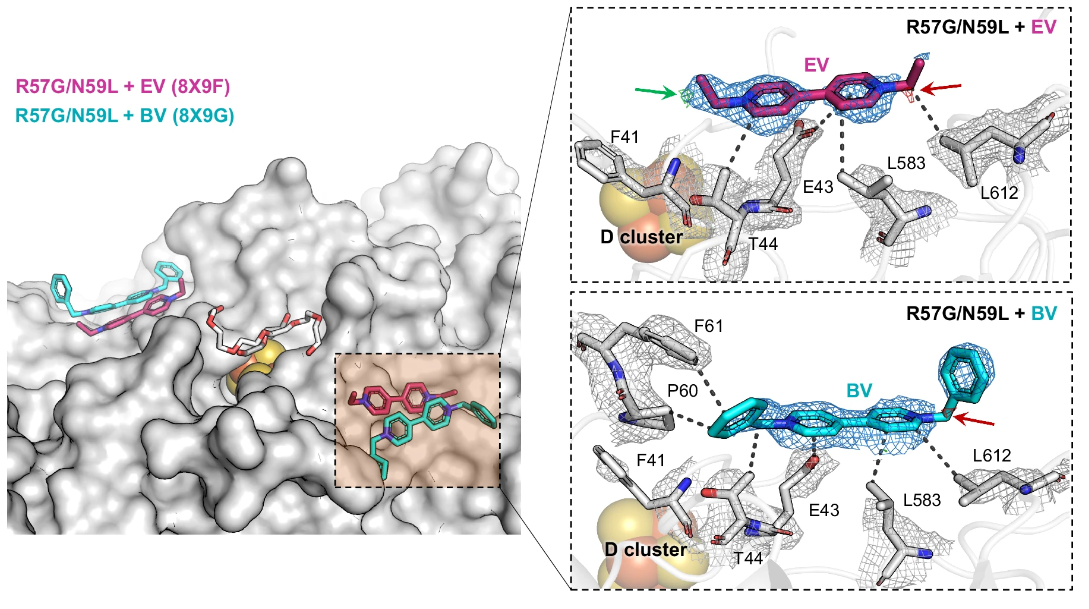
Figure 2. A schematic image, showing the crystal structures of the R57G/N59L variant in complex with EV and BV (PDB ID: 8X9F and 8X9G, respectively).
Moreover, the research team developed a method for precisely manipulating specific parts of the enzyme to enhance its activity. By introducing mutations to the enzyme’s electron transport site and replacing it with another amino acid, the team was able to improve the enzyme’s affinity with the electron transport medium. This advancement enables the enzyme to purify gases more quickly and efficiently than before.
Professor Yong Hwan Kim expressed his excitement about the potential impact of this research, stating, “It has opened up new possibilities for industrial waste hydrolysis technology using enzymes.” He further added, “Our experiments have demonstrated the high purification capacity of this enzyme for actual industrial waste gas.”

Figure 3. (Left) A schematic for CODH enzyme reactions using industrial off-gases and plastic waste syngas. (Right) Influence of gas mixtures on ChCODH2 R57G/N59L/A559W. The activity of the mutant was measured using industrial flue gas. The relative activity was compared to the enzyme activity with pure CO as a standard. The constituents of each gas mixture are depicted within the bars. White dots for each point overlay bar charts.
Meanwhile, Research Professor Suk Min Kim, the first author and corresponding author of the study, emphasized the practical applications of their findings. “The results of this study can be utilized in the production of valuable chemicals by utilizing waste gas from the steel industry as a carbon resource,” he explained. This innovation is expected to significantly contribute to achieving carbon neutrality in Korea.
Published in the online version of Nature Communications on March 28, this groundbreaking research provides a novel solution to the environmental pollution issues associated with industrial waste gas. By addressing both sustainable environmental protection and industrial development, this study marks a significant milestone in the field. The study has been supported by the C1 Gas Refinery Program and the Engineering Research Center Program through the National Research Foundation (NRF) of Korea, funded by the Ministry of Science and ICT (MSIT).
Journal Reference
Suk Min Kim, Sung Heuck Kang, Jinhee Lee, et al., “Identifying a key spot for electron mediator-interaction to tailor CO dehydrogenase’s affinity,” Nat. Commun., (2024).