A new coating for lithium-ion batteries (LIBs), developed by scientists at UNIST promises extended driving for future electric vehicles (EVs). The coating, described in a paper published in the journal Nature Energy, when applied to LIBs is shown to have improved cycling stability even after being charged and discharged more than 500 times. As a result, the development of EV batteries that can drive longer distances with a single battery charge has gained considerable momentum.
Distinguished Professor Jaephil Cho and his research team in the School of Energy and Chemical Engineering at UNIST unveiled a new coating technology that can greatly suppress intergranular cracking, chemical side reactions, and impedance growth. According to the research team, this is extraordinary given that it happens at room temperature, and the secondary particle does not alter the crystalline bulk, but produces drastic changes in the grain boundaries (GBs) as they are infiltrated by reactive wetting.
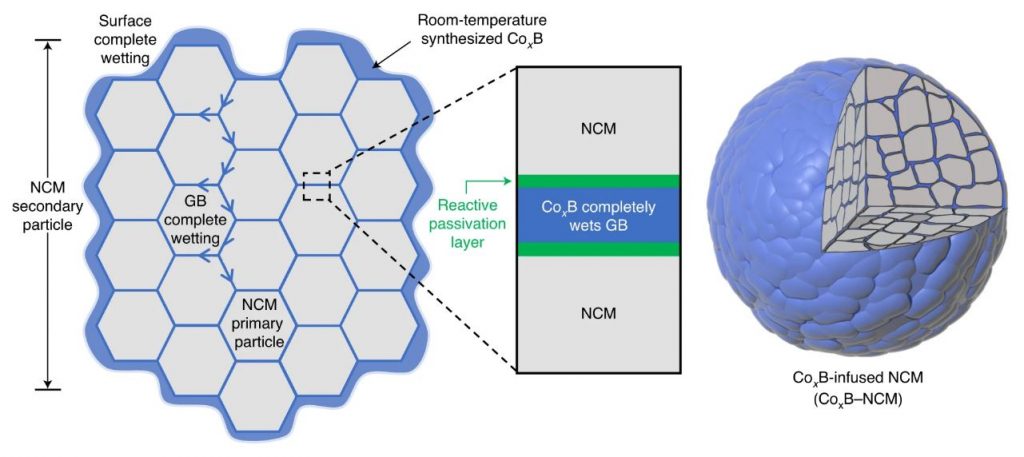
Figure 1. Schematic coating-plus-infusion microstructure in which CoxB uniformly coats the surface of NCM secondary particle and infuses into GBs between the NCM primary particles.
Nickel-rich (Ni-rich) materials are considered promising cathode materials, as they can deliver higher capacity at lower costs. However, conventional Ni-rich cathodes have been limited in terms of short lifespans, caused by microcracking and the side reactions of the electrolyte due to repeated charging/discharging operation. For this reason, in order to prevent electrolyte degradation, a protective coating is being applied onto the surfaces of all materials that are currently being produced with heat treatment at 700°C or higher. However, there had been problems with poor performance and high production costs.
In the study, the research team has presented a room-temperature synthesis route to achieve a full surface coverage of secondary particles and facile infusion into grain boundaries, and thus offer a complete ‘coating-plus-infusion’ strategy. Through this method, they constructed a high-quality cobalt boride (CoxB) metallic glass infusion of NCM secondary particles by reactive wetting. Under the strong driving force of an interfacial chemical reaction, nanoscale cobalt boride (CoxB) metallic glass not only completely wraps around the secondary particle surfaces, but also infuses into the grain boundaries (GBs) between primary particles. This is extraordinary given that it happens at room temperature, and the secondary particle does not alter the crystalline bulk, but produces drastic changes in the GBs as they are infiltrated by reactive wetting. Consequently, it offers superior electrochemical performance and better safety by mitigating the entwined cathode-side intergranular SCC, microstructural degradation, and side reactions, as well as the TM crossover effect to the anode.
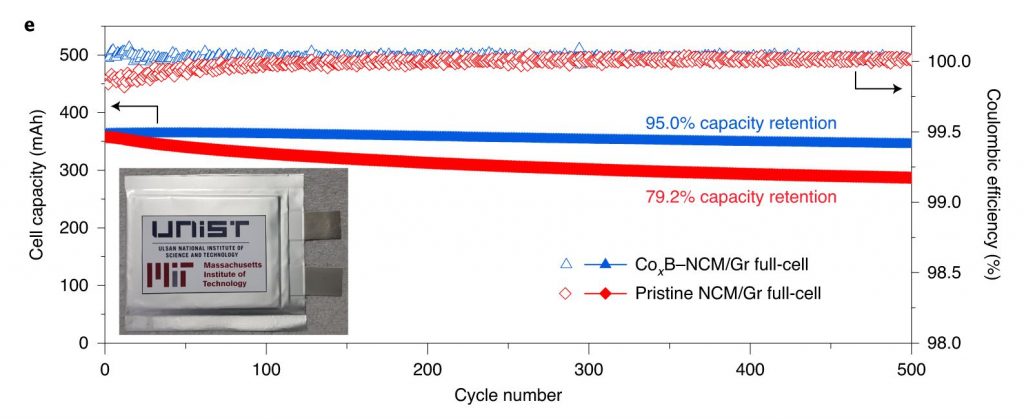
Figure 2. Superior electrochemical performance of CoxB–NCM over pristine NCM. (e) Cycling performance of CoxB–NCM/Gr and pristine NCM/Gr full-cells at 1.0C in the range of 2.8−4.3 V at 25 °C. Inset: photo of an assembled pouch cell.
Their findings reveal that the battery, constructed with the new coating method exhibited an impressive 95% capacity retention over 500 cycles, which is about 20% improved life retention rate compared to the existing Ni-rich materials. Not only that it has also dramatically improved the rate capability and cycling stability of NCM, including under high-discharge rate and high-temperature (45 °C) conditions, as it greatly suppressed intergranular cracking, side reactions and impedance growth.
The findings of this research have been published in the March 2021 issue of Nature Energy. It has been carried out in collaboration with Professor Ju Li’s research group from the MIT Department of Materials Science and Engineering.
Journal Reference
Moonsu Yoon, Yanhao Dong, Jaeseong Hwang, et al., “Reactive boride infusion stabilizes Ni-rich cathodes for lithium-ion batteries,” Nature Energy, (2021).












