A team of researchers, jointly led by Professor Hyun-Wook Lee and Professor Dong-Hwa Seo from the School of Energy and Chemical Engineering at the Ulsan National Institute of Science and Technology (UNIST), in collaboration with Professor Seok Woo Lee from Nanyang Technological University in Singapore, has achieved significant breakthroughs in harnessing low-grade heat sources (<100 °C) for efficient energy conversion. Their groundbreaking work focuses on developing a highly efficient Thermally Regenerative Electrochemical Cycle (TREC) system capable of converting small temperature differences into usable energy.
Conventional energy-harvesting systems face challenges when it comes to effectively utilizing low-grade heat sources. However, TREC systems offer an attractive solution as they integrate battery functionality with thermal-energy-harvesting capabilities. In this study, the research team delved into the role of structural vibration modes to enhance the efficacy of TREC systems.
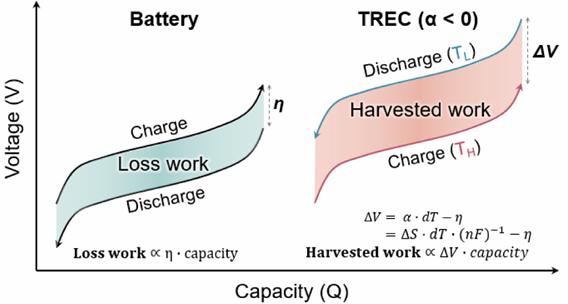
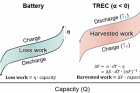
Figure 1. Schematic showing the different mechanisms of a battery and TREC system. Whereas the battery system (left) loses some of the stored energy as unusable energy, the TREC system (right) can convert low-grade waste-heat energy into electrochemical energy during battery cycling.
By analyzing how changes in covalent bonding influence vibration modes—specifically affecting structural water molecules—the researchers discovered that even minute amounts of water induce strong structural vibrations within cyanide ligands’ A1g stretching mode. These vibrations substantially contribute to a larger temperature coefficient (ɑ) within a TREC system. Based on these insights, the team designed and implemented a highly efficient TREC system using a sodium-ion-based aqueous electrolyte.
“This study provides valuable insights into how structural vibration modes can enhance the energy-harvesting capabilities of TREC systems,” explained Professor Hyun-Wook Lee. “Our findings deepen our understanding of Prussian Blue analogs’ intrinsic properties regulated by these vibration modes—opening up new possibilities for improved energy conversion.”
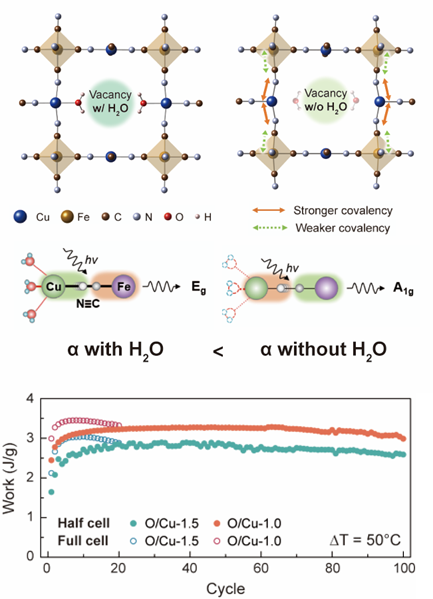 Figure 2. Principle of TREC and effect of water molecules in a PBA structure. (Top) Effect of removal of water molecules on the CuHCFe structure and covalency variation (-ICOHP/eV). The average -ICOHP values of Cu─N and Fe─C bonds and the SD of -ICOHP values of 6 Fe─C bonds are presented. (Center) Effect of water molecules on the stretching vibration mode of cyanide ligands. (Bottom) d) Amount of harvested work as a result of TREC full-cell and half-cell. The low and high temperatures are 10 and 60 °C, respectively. The current density of the full cell is set at 0.5 C (30 mA g−1) based on O/Cu-x.
Figure 2. Principle of TREC and effect of water molecules in a PBA structure. (Top) Effect of removal of water molecules on the CuHCFe structure and covalency variation (-ICOHP/eV). The average -ICOHP values of Cu─N and Fe─C bonds and the SD of -ICOHP values of 6 Fe─C bonds are presented. (Center) Effect of water molecules on the stretching vibration mode of cyanide ligands. (Bottom) d) Amount of harvested work as a result of TREC full-cell and half-cell. The low and high temperatures are 10 and 60 °C, respectively. The current density of the full cell is set at 0.5 C (30 mA g−1) based on O/Cu-x.
The potential applications for TREC systems are vast, particularly in wearable technologies and other devices where small temperature differentials exist. By effectively capturing and converting low-grade heat into usable energy, TREC systems offer a promising pathway towards the development of next-generation secondary batteries.
The study findings have been published ahead of their official publication in the online version of Advanced Materials on July 3, 2023. This research has received support from the 2023 Research Fund of UNIST, Individual Basic Science & Engineering Research Program, and the National Center for Materials Research Data through the National Research Foundation (NRF) of Korea, funded by the Ministry of Science and ICT (MSIT).
Journal Reference
Ahreum Choi, You-Yeob Song, Juyoung Kim, et al., “Enhancing Efficiency of Low-Grade Heat Harvesting by Structural Vibration Entropy in Thermally Regenerative Electrochemical Cycles,” Adv. Mater., (2023).