A groundbreaking development in biomedical engineering has led to the creation of a human stomach micro-physiological system (hsMPS), representing a significant leap forward in understanding and treating various gastrointestinal diseases, including stomach cancer. The research team, led by Professor Tae-Eun Park from the Department of Biomedical Engineering at UNIST and Professor Seong-Ho Kong from Seoul National University Hospital, has successfully developed a biomimetic chip that combines organoid and organ-on-a-chip technologies to simulate the complex defense mechanisms of the human gastric mucosa.
Organoids, which mimic human organs using stem cells, have shown great potential as in vitro models for studying specific functions. However, they lack the ability to replicate mechanical stimulation or cell-to-cell interactions found within the human body. This limitation prompted researchers to develop an innovative biochip capable of recreating real-life gastric mucosal protection systems.
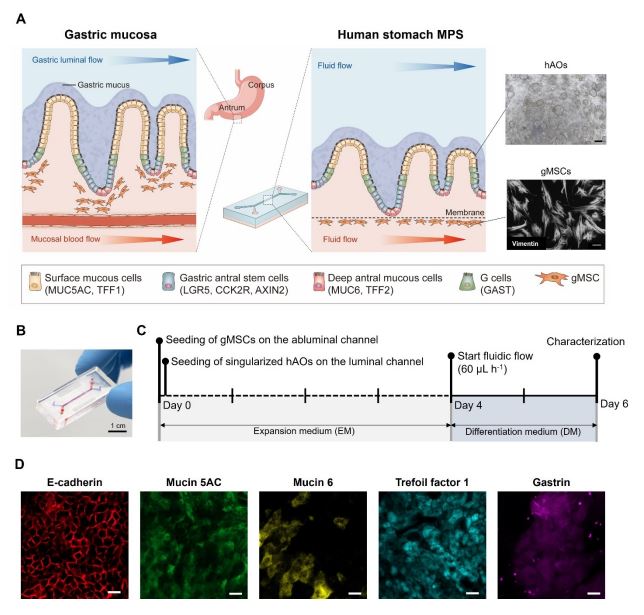 Figure 1. Reconstitution of the human stomach in an MPS device. (A) Schematic illustration of the human gastric mucosa (left) and the human stomach MPS (hsMPS) with singularized human gastric antral organoid (hAOs) cultured on the apical luminal channel interfaced with primary gastric stromal cells (gMSCs) on the basal abluminal channel (center). At the top right, a bright field image of hAOs (bar, 250 μm) used to generate gastric epithelium, and at the bottom right, an immunofluorescence micrograph of the gMSCs labelled with Vimentin (bar, 100 μm) are shown. (B) Photograph of the hsMPS (bar, 1 cm). (C) Timeline for the reconstitution of the human stomach in an MPS device. (D) Immunofluorescence micrographs of the gastric epithelium in the hsMPS at 6 days labelled with E-cadherin, mucin 5AC, mucin 6, trefoil factor 1, and gastrin (bar, 20 μm).
Figure 1. Reconstitution of the human stomach in an MPS device. (A) Schematic illustration of the human gastric mucosa (left) and the human stomach MPS (hsMPS) with singularized human gastric antral organoid (hAOs) cultured on the apical luminal channel interfaced with primary gastric stromal cells (gMSCs) on the basal abluminal channel (center). At the top right, a bright field image of hAOs (bar, 250 μm) used to generate gastric epithelium, and at the bottom right, an immunofluorescence micrograph of the gMSCs labelled with Vimentin (bar, 100 μm) are shown. (B) Photograph of the hsMPS (bar, 1 cm). (C) Timeline for the reconstitution of the human stomach in an MPS device. (D) Immunofluorescence micrographs of the gastric epithelium in the hsMPS at 6 days labelled with E-cadherin, mucin 5AC, mucin 6, trefoil factor 1, and gastrin (bar, 20 μm).
The newly developed biochip incorporates fluid flow within its microfluidic channels to simulate mechanical stimuli and facilitate cell-to-cell interactions. Mesenchymal substrate cells exposed to fluid flow activate gastric stem cell proliferation while promoting cellular differentiation balance. This process ultimately mimics key features necessary for developing functional gastric mucosal barriers at a biologically relevant level.
One remarkable achievement demonstrated by this hsMPS is its ability to uncover previously unseen defense mechanisms against Helicobacter pylori—a pathogen associated with various stomach diseases—in ways that were not possible with existing models. Gastric mucosal peptide known as TFF1 was observed forming mosaic-like structures within groups infected with Helicobacter pylori—forming a protective barrier essential for establishing an efficient defense system against external infectious factors. Suppression of gastric mucosal peptide expression resulted in more severe inflammatory reactions.
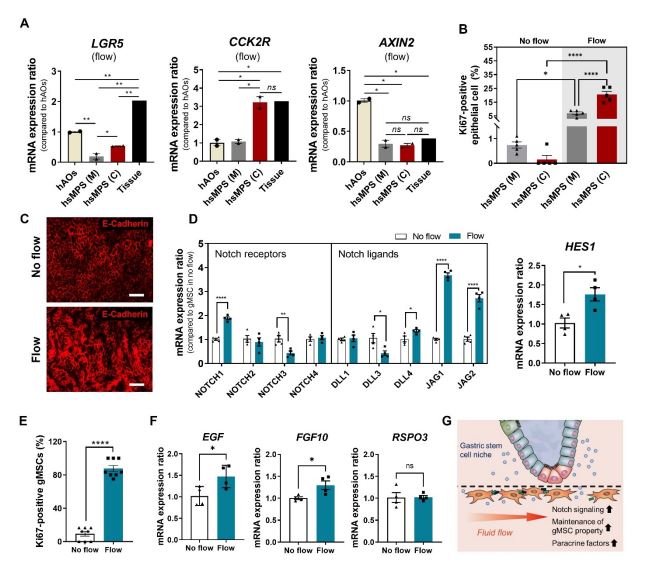 Figure 2. Gastric epithelial mitotic activity and morphogenesis in the hsMPS.
Figure 2. Gastric epithelial mitotic activity and morphogenesis in the hsMPS.
“This study presents our model’s potential for observing dynamic interactions between epithelial cells and immune cells in chips infected with Helicobacter pylori, contributing to a comprehensive understanding of gastric mucosal barrier stability,” explained Professor Park.
The research findings, supported by the Basic Research Laboratory (BRL) research grant from the National Research (NRF), funded by the Ministry of Science and ICT (MSIT), have been published online on July 31 in Advanced Science—a prestigious journal published by Wiley.
These groundbreaking advancements in hsMPS open up new avenues for studying host-microbe interactions, developing therapeutic strategies for gastric infections, and gaining a deeper understanding of gastrointestinal diseases. This innovative biochip technology has the potential to reduce reliance on animal experimentation while providing valuable insights into complex physiological processes within the human stomach.
Journal Reference
Hye-Jin Jeong, Ji-Hyeon Park, Joo H. Kang, et al., “Organoid-based human stomach micro-physiological system to recapitulate the dynamic mucosal defense mechanism,” Adv. Sci., (2023).