A recent study, affiliated with UNIST has developed a system that produces electricity and hydrogen (H2) while eliminating carbon dioxide (CO2), which is the main contributor of global warming.
Published This breakthrough has been led by Professor Guntae Kim in the School of Energy and Chemical Engineering at UNIST in collaboration with Professor Jaephil Cho in the Department of Energy Engineering and Professor Meilin Liu in the School of Materials Science and Engineering at Georgia Institute of Technology.
In this work, the research team presented Hybrid Na-CO2 system that can continuously produce electrical energy and hydrogen through efficient CO2 conversion with stable operation for over 1,000 hr from spontaneous CO2 dissolution in aqueous solution.
“Carbon capture, utilization, and sequestration (CCUS) technologies have recently received a great deal of attention for providing a pathway in dealing with global climate change,” says Professor Kim. “The key to that technology is the easy conversion of chemically stable CO2 molecules to other materials.” He adds, “Our new system has solved this problem with CO2 dissolution mechanism.”
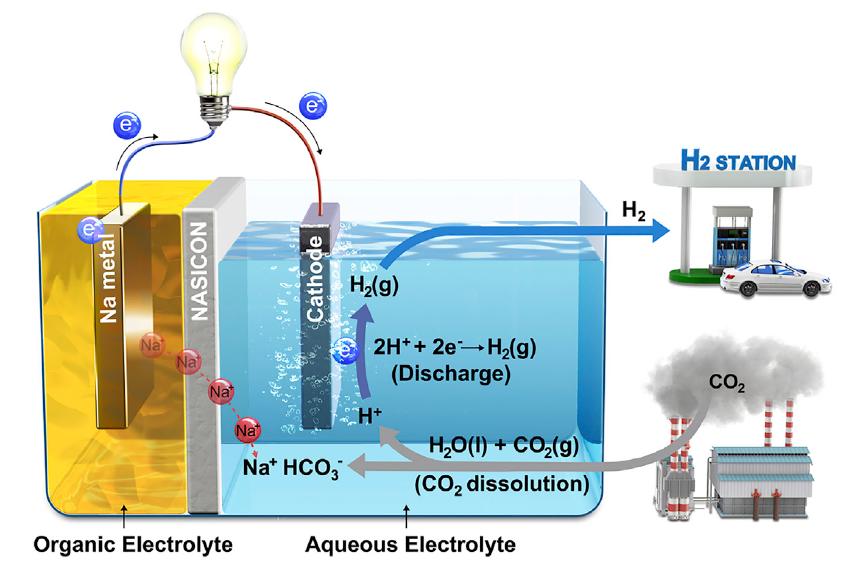
Schematic illustration of Hybrid Na-CO2 System and its reaction mechanism.
Much of human CO2 emissions are absorbed by the ocean and turned into acidity. The researchers focused on this phenomenon and came up with the idea of melting CO2 into water to induce an electrochemical reaction. If acidity increases, the number of protons increases, which in turn increases the power to attract electrons. If a battery system is created based on this phenomenon, electricity can be produced by removing CO2.
Their Hybrid Na-CO2 System, just like a fuel cell, consists of a cathode (sodium metal), separator (NASICON), and anode (catalyst). Unlike other batteries, catalysts are contained in water and are connected by a lead wire to a cathode. When CO2 is injected into the water, the entire reaction gets started, eliminating CO2 and creating electricity and H2. At this time, the conversion efficiency of CO2 is high at 50%.
“This hybrid Na-CO2 cell, which adopts efficient CCUS technologies, not only utilizes CO2 as the resource for generating electrical energy but also produces the clean energy source, hydrogen,” says Jeongwon Kim in the Combined M.S/Ph.D. in Energy Engineering at UNIST, the co-first author for the research.
▲ The system produce electrical energy and hydrogen through efficient CO2 conversion (Electric current is presented on the display below).
In particular, this system has shown stability to the point of operating for more than 1,000 hours without damage to electrodes. The system can be applied to remove CO2 by inducing voluntary chemical reactions.
“This research will lead to more derived research and will be able to produce H2 and electricity more effectively when electrolytes, separator, system design, and electrocatalysts are improved,” said Professor Kim.
Journal Reference
Changmin Kim et. al., “Efficient CO2 Utilization via a Hybrid Na-CO2 System Based on CO2 Dissolution,” iScience, (2018).