A research team, affiliated with UNIST has achieved a groundbreaking feat by observing the dissolution of salt in water at the atomic level and experimentally uncovering the underlying principle.
Led by Professor Hyung-Joon Shin and his researchers from the Department of Materials Science and Engineering at UNIST, the team introduced the innovative “single ion control technology.” This cutting-edge approach enables the precise manipulation of individual water molecules to selectively extract specific ions from salt.
Figure 1. Schematic images of salt melting in water and dissolution of a single ion observed at atomic level.
Salt, composed of robust ionic bonds between sodium cations (Na+) and chlorine anions (Cl-), undergoes a transformative process when immersed in water. The interaction between the positive and negative polarities of water molecules disrupts the bond between the sodium and chlorine ions, leading to their separation and the formation of saltwater.
While the principle of salt dissolution in water may seem straightforward, previous studies have predominantly explored this phenomenon theoretically. However, the ability to experimentally confirm which ions dissolve first in water and elucidate the mechanism by which water molecules weaken the ionic bonds of salt had remained elusive until now. Professor Shin remarked, “The challenge lay in the intricate nature of studying and controlling individual ions amidst the dynamic movement of dissolved ions within water.”
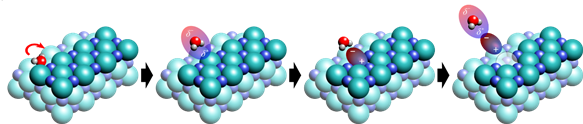
Figure 2. Schematic image, showing the real-space observation of selective dissolution of salt by manipulating a single water molecule.
In a meticulously controlled experiment conducted in cryogenic and ultra-high vacuum conditions at -268.8°C, the research team placed a water molecule on a thin salt membrane consisting of two to three atomic layers. Employing a scanning tunneling microscope (STM) capable of atomic-scale measurements, the team observed a minute change in height of 10 picometers (pm) as water molecules were maneuvered horizontally across the salt membrane. This observation was attributed to the strong interaction between chlorine anions and water molecules.
By strategically moving water molecules along the salt film with varying atomic thickness, the researchers successfully induced the disappearance of a chlorine anion from its path. The polarity of water molecules played a pivotal role in breaking the ionic bond of salt, causing the chlorine anion to emerge ahead of the sodium cation.
The team further highlighted that the polarization rate of chlorine anions, which are 20 times more pronounced than sodium cations, renders them highly sensitive to external electrical changes induced by water molecules. This heightened responsiveness was particularly evident in regions where atoms lacked sufficient bonding with the surrounding environment.
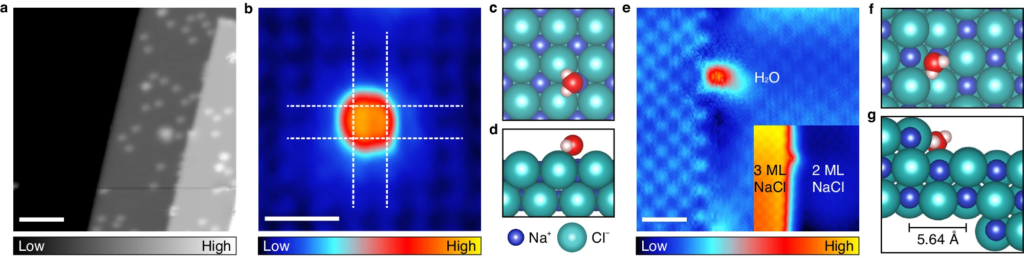
Figure 3. Water molecules on a NaCl surface.
Huijun Han (Combined MS/Ph.D. Program of Materials Science and Engineering, UNIST), the lead author of the paper expressed, “While the theoretical understanding of salt melting in water has long been established, our success in extracting single ions through precise water molecule control marks a significant experimental breakthrough.”
“Ions play a pivotal role in altering the performance of batteries and semiconductor materials,” Professor Shin emphasized. “We envision leveraging the single ion control technology to advance fundamental technologies related to ion functionalities.”
The findings of the study have been published in the online version of Nature Communications on March 16, 2024. This study was jointly participated in by Professor Feng Ding from the Shenzhen Institutes of Advanced Technology (SIAT) of the Chinese Academy of Science (CAS) and received support from the National Research Foundation of Korea (NRF), the Ministry of Science and ICT (MSIT), and the Institute for Basic Science (IBS).
Journal Reference
Huijun Han, Yunjae Park, Yohan Kim, et al., “Controlled dissolution of a single ion from a salt interface,” Nat. Commun., (2024).












