UNIST has recently announced that the institution’s In Vivo Research Center (IVRC) became the first laboratory in the nation’s southeastern region to receive a Certificate of Good Laboratory Practice (GLP) compliance from the Ministry of Food and Drug Safety (MFDS), formerly known as the Korea Food & Drug Administration (KFDA). The certificate acknowledges the site is operating in compliance with OECD Principles of GLP for both toxicology and laboratory services.
Currently, there are eight institutions in South Korea that have KDFA-designated animal testing laboratories. UNIST is the only institution in South Korea to have a good animal testing facility without having a medical school.
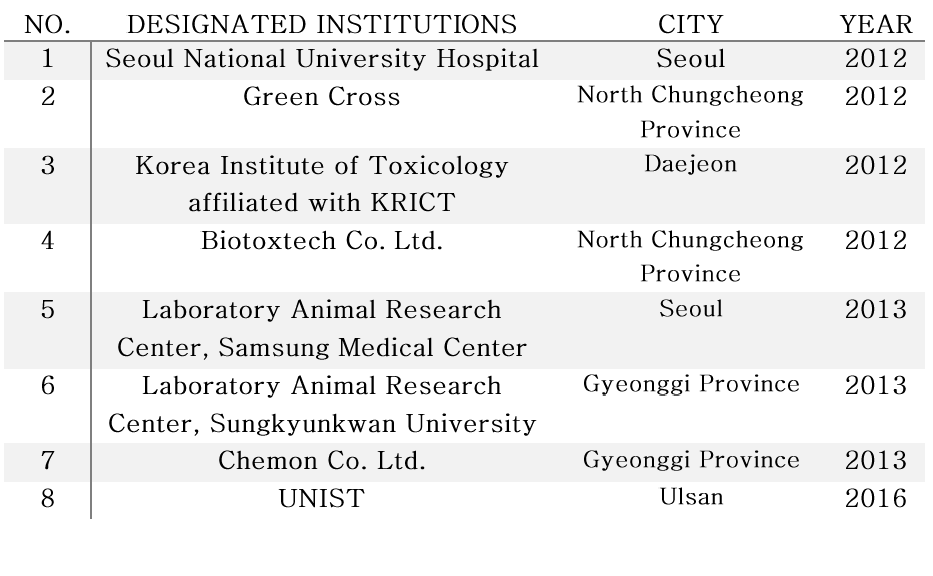
▲ Current state of GLP-designated institutions (As of 2016).
In order to receive the KDFA Good Laboratory Practices (GLP) recognition, an institution must meet the following requirements. First is to secure at least one veterinarian and an expert with at least 3 years of practical work in the area of laboratory animal science. On the facility side, one must have the following facilities, such as a breeding room, a laboratory, a quarantine room, an operating room, an autopsy room, as well as a waste storage room.
This certificate is recognized throughout South Korea and it took the workers of the facility more than three years of intense work to gain the certificate.
Established in 2013, the In Vivo Research Center (IVRC) located in the UNIST Central Research Facilities (UCRF) offers strict management for clean breeding, as well as the state-of-the-art equipment support for bio-efficacy analysis. The center also conducts research on Alzheimer’s, cancer, as well as metabolic diseases, such asdiabetes and obesity. The space of IVRC is composed of four animal zones and four efficacy analysis lab for the systematic classification and functional management in accordance with the purpose of animals use.

A researcher is examining the laboratory mice at the husbandry, UNIST.
Director Hyeon Suk Shin of UCRF states, “This facility will help UNIST improve the quality and reliability of activities in life sciences and related industries.” He adds, “Moreover, this recognition will develop new research opportunities and put UNIST at the heart of South Korea’s thriving life sciences industry.”
The IVRC was also selected as the best research facility, providing a safe laboratory environment for its researchers and students and given a condemnation of Minister Prize Award from the Korean Ministry of Science, ICT and Future Planning. When inspected by the Living Modified Organisms (LMOs) Laboratory Safety Management Center, UNIST’s IVRC was rated as a safe place to carry out experiments involving LMOs.









![[2026 Matriculation] UNIST Welcomes Class of 2030!](https://news.unist.ac.kr/wp-content/uploads/2026/02/사진-박종래-UNIST-총장이-2026년-입학식사를-전하고-있다-2-190x122.jpg)
![[2026 UNIST Commencement] UNIST Confers Degrees to 883 Graduates](https://news.unist.ac.kr/wp-content/uploads/2026/02/사진-2026학년도-UNIST-졸업생들이-학사모를-위로-던지며-졸업을-축하하고-있다-1-800x413-190x122.jpg)



