A recent study, led by Professor JaePhil Cho in the School of Energy and Chemical Engineering at UNIST has unveiled a new anode material, designed to help speed up fast charging in new electric cars with longer range. The key is to make many channels through which graphite can pass lithium ions quickly, and thinly coat silicon with nano-thickness on it. Applying this technology to future electric vehicle batteries will contribute to shortening charging time and increasing mileage.
It has become important to increase the capacity of the secondary battery, which is a power source, while reducing the charging time while paying attention to electric vehicles. However, existing graphite cathode materials have a small capacity to store lithium ions, and when charged at high speed, lithium metal precipitates on the surface of the cathode material, deteriorating battery performance and safety.
As a material to overcome the disadvantages of graphite anode materials, silicon materials, which are 10 times larger than graphite, are attracting attention as next generation anode materials. Silicon materials, however, have a low electrical conductivity and a rapid change in volume during charging and discharging. For this reason, the technology to make lithium ion batteries that simultaneously realize high capacity and fast charging has been a difficult problem until now.
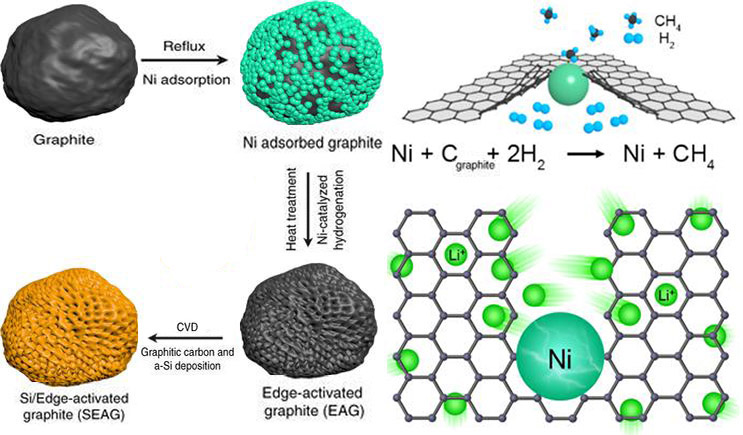
Schematic of the procedures for fabrication and characterization of SEAG.
In this study, Professor Cho and his research team proposed a method to synthesize a graphite / silicon composite with a new structure to overcome the limitations of existing cathode materials. The ‘edge activated graphite / silicon composite’ synthesized by this method was charged 1.5 times faster under commercialized electrode conditions and the capacity increased by 50%.
demonstrated a hybrid anode via incorporation of a uniformly implanted amorphous silicon nanolayer and edge-site-activated graphite
“At the core of this technology is a ‘nickel catalyst reduction method’ that creates highways where lithium ions can be rapidly loaded into graphite and a ‘silicon nano coating’ that increases the capacity stably,” says Namhyung Kim in the Combined M.S./Ph.D. of Energy and Chemical Engineering, the first author of the study. “The new materials have problems with existing graphite and silicon “We have demonstrated the possibility of applying it to next-generation high-capacity cathode materials.”
Nickel can act as a catalyst to react carbon and hydrogen. Using this point, the researchers attached nickel to the edges of the graphite cathode material and reacted with hydrogen. As a result, the carbon at the edges of the graphite has turned into methane (CH 4 ) gas. When these reactions occur consecutively, a hole is formed in the edge of the graphite, and a path through which lithium ions can easily enter is opened. As the number of such passageways increases, the battery can be charged quickly as a result.
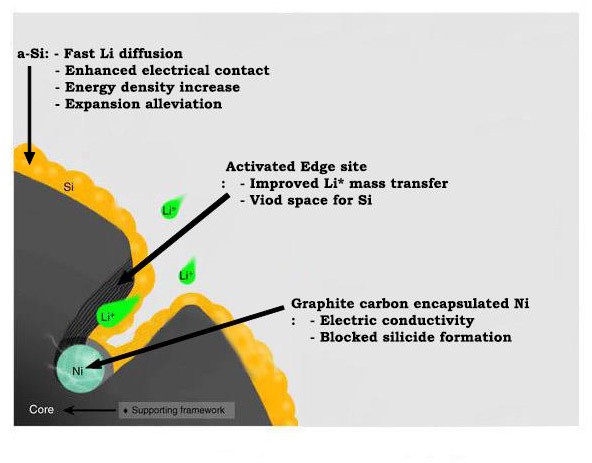
Cross-sectional illustration showing the detailed structural characteristics of SEAG.
The researchers also fabricated a structure that thinly coated silicon with perforated graphite to increase capacity over existing graphite cathode materials. It is a combination of high conductivity of graphite and high capacity of silicon.
“Silicon nanoclay technology is used to coat high-performance graphite / silicon composites evenly on the surface of graphite with silicon (about 20 nm or less) of the hair,” says Professor Cho. “The whole process is relatively simple and cheap, so it is possible to mass-produce it.”
He adds, “This research can be successfully applied to cathode materials used in high-energy, high-power batteries, such as electric vehicles and high-energy storage (ESS).”
The findings of the study has been published online in the October 9 issue of the journal Nature Communications. The research was carried out with the support of Samsung Electronics Future Technology Development Center.
Journal Reference
Namhyung Kim, et. al., “Fast-charging High-energy Lithium-ion Batteries via Implantation of Amorphous Silicon Nanolayer in Edge-plane Activated Graphite Anodes”, Nature Communications, (2017).