A team of researchers, affiliated with UNIST has identified a new intracellular trafficking pathway of phospholipids. This pathway serves as a tunnel through which lipids are transported at inter-organellar membrane contact sites (MCSs). The discovery has great significance in the study of human diseases associated with defects in phospholipid transport.
This study has been led by Professor Changwook Lee in the School of Life Sciences at UNIST. In the study, the research team has identified the trafficking mechanism of phospholipids that occurs at MCSs between the ER and mitochondria within eukaryotic cells that compose humans and other higher animals.
In addition to the nucleus, eukaryotic cells may contain several other types of organelles, including mitochondria, the ER, and lysosomes. These organelles exchange cellular materials through small sacs, known as transport vesicles and this is essential for cell survival. However, new information about the nonvesicular lipid trafficking occurring at the ER-mitochondria MCS has caused researchers to carry out further research in this area.
In the study, Professor Lee’s team has focused on the exchange of cellular materials between the ER and mitochondria, as these two organelles produce phospholipids without having vesicles during the exchange of materials. 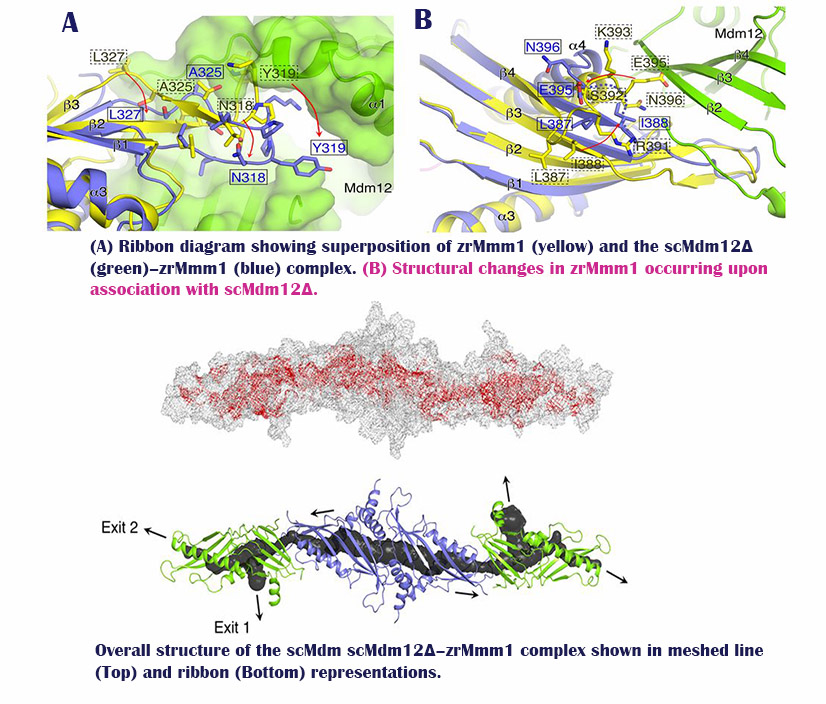
“Newly-synthesized phosphatidylserine (PS) is transported from the ER to mitochondria and subsequently decarboxylated to become phosphatidylethanolamine (PE) by the inner membrane-resident enzyme. This is then converted to phosphatidylcholine (PC) in the ER,” says Hanbin Jeong in the Combined M.S/Ph.D. of Life Sciences, the first author of the study. “Thus, phospholipid synthesis is important to transfer phospholipids between two spatially separated organelles.”
Thus, phospholipids must be transported from their sites of synthesis to the membranes of other organelles in some way. However, the details regarding the mechanism of the exchange of materials between these two organelles are still unknown.
The researchers found a solution in the ER-mitochondria encounter structure (ERMES) complex comprising the ER proteins Mmm1 and cytosolic Mdm12 and the mitochondria proteins Mdm34 and Mdm10.
“By analyzing the Mdm12/Mmm1 complex via X-ray crystallography, we have discovered a three-dimensional pathway structure, in which phospholipids, such as PS, PE, and PC are transported in between the ER and mitochondria,” says Professor Lee. “Such pathway, created by the combination of two proteins create hydrophobic environments and serves as a pathway for phospholipid transfer.”
“Our study, which reveals the structure and principle of phospholipid transport between the ER and mitochondria, would be a valuable resource to help understand the origin of life,” says Professor Lee. “This will provide a new theoretical foundation for the treatment of diseases associated with defects in phospholipid transport.”
The findings of the study have been published in the journal PNAS on October 25, 2017. This research was supported by the Cell Logistics Research Center, the Basic Research Program, and the Global Ph.D. Fellowship Program Grant from the National Research Foundation of Korea. This work was also supported by the Institute for Basic Science
Journal Reference
Hanbin Jeong, et al., “Crystal structures of Mmm1 and Mdm12–Mmm1 reveal mechanistic insight into phospholipid trafficking at ER-mitochondria contact sites,” (2017), PNAS.