A research team, affiliated with UNIST has announced that they have successfully developed a high polymer binder material that helps catalysts stick to electrodes of seawater batteries to improve performance. The adhesive material was designed after sticky protein, produced by mussels, that sticks to various surfaces including rocks, boats, and ropes.
This breakthrough has been led by Professor Dong Woog Lee in the School of Energy and Chemical Engineering at UNIST. Published in the Journal of the Journal of materials chemistry A (JMCA), their findings have been featured on the outside cover of the printed edition on March 7, 2022. It has also been jointly participated by Professor Hyun-Wook Lee and Professor Sang Kyu Kwak in the School of Energy and Chemical Engineering at UNIST.

Figure 1. Schematics showing the triple-phase contact zone of the SAB battery. Schemes of (a) air-electrode-containing polymer binder, Pt/C catalyst, and carbon current collector (heat-treated carbon felt, HCF) in aqueous media, and (b) design of hydrophilic catechol polymer binders.
Seawater batteries use a liquid solution of saltwater to collect, store and discharge electricity. Their cathodes consist of current collectors made of carbon fiber and catalyst materials. Catalysts help batteries store and discharge energy.
Normally, polyvinylidene difluoride (PVDF), a highly non-reactive thermal plastic fluoropolymer, is used as a binder for catalysts but PVDF’s performance is greatly reduced in seawater. When catalysts are not properly adhered to the current collector, the corrosion of carbon materials takes place due to overvoltage.
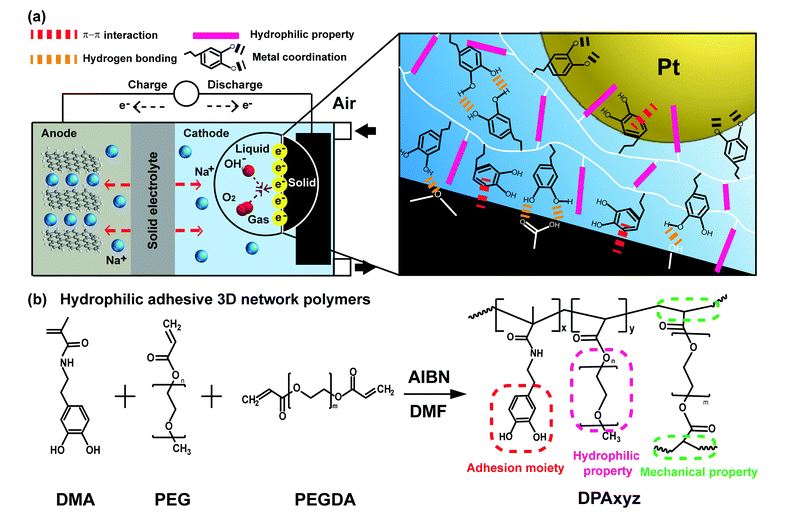
Figure 2. Schematics showing the triple-phase contact zone of the SAB battery. Schemes of (a) air-electrode-containing polymer binder, Pt/C catalyst, and carbon current collector (heat-treated carbon felt, HCF) in aqueous media, and (b) design of hydrophilic catechol polymer binders.
Researchers said that its research team has developed an underwater binder that mimics the protein-based glue produced by mussels. Researchers found that a seawater battery using the newly-developed binder showed up to 60 percent less occurrence of overvoltage. The performance of electrodes was improved by about 400 percent.
“The biomimetic binder protects current collectors from corrosion and catalysts from breaking apart,” said Jieun Choi, the co-author of the study. “This research will contribute to the early commercialization of seawater batteries and other aqueous metal-air batteries.”
The study has been supported by the Korea Hydro & Nuclear Power Co., Ltd. and the National Research Foundation (NRF) of Korea.
Journal Reference
Myung-Jin Baek, Jieun Choi, Tae-Ung Wi et al., “Strong Interfacial Energetics Between Catalysts and Current Collectors in Aqueous Sodium–air Batteries,” J. Mater. Chem. A., (2022).