A research team, affiliated with UNIST, has made a significant breakthrough in uncovering a novel mechanism to regulate cell death in cancer cells. This discovery provides new insights to improve our understanding of cancer occurrence. This breakthrough has been led by Professor Chan Young Park and his research team from the Department of Biological Sciences at UNIST.
Normal cells undergo apoptosis when exposed to stressful situations like nutrient deficiency or hypoxia. However, cancer cells have developed resistance against programmed cell death and constantly proliferate by developing mechanisms that are favorable for their survival. Among these mechanisms, a non-apoptotic cell death process, also known as Entosis has recently been reported as an alternative pathway for regulating cellular processes in invasive and predatory cancer cells.
Entosis is a form of cell predation, wherein invasive cancer cells invade other cancer cells to create a “cell-in-cell” structure. This process allows for the creation of an advantageous environment that can be avoided through entotic cell death. Predatory cells receive nutrients and chromosomes from invasive cells, while invasive cells hide in predatory cells and divide within them when appropriate conditions arise. Such occurrences affect the development of cancer by causing genetic instability such as abnormalities in chromosome numbers due to interactions between cancerous cells. Therefore, understanding Entosis is essential for studying the causes and treatments of cancer.
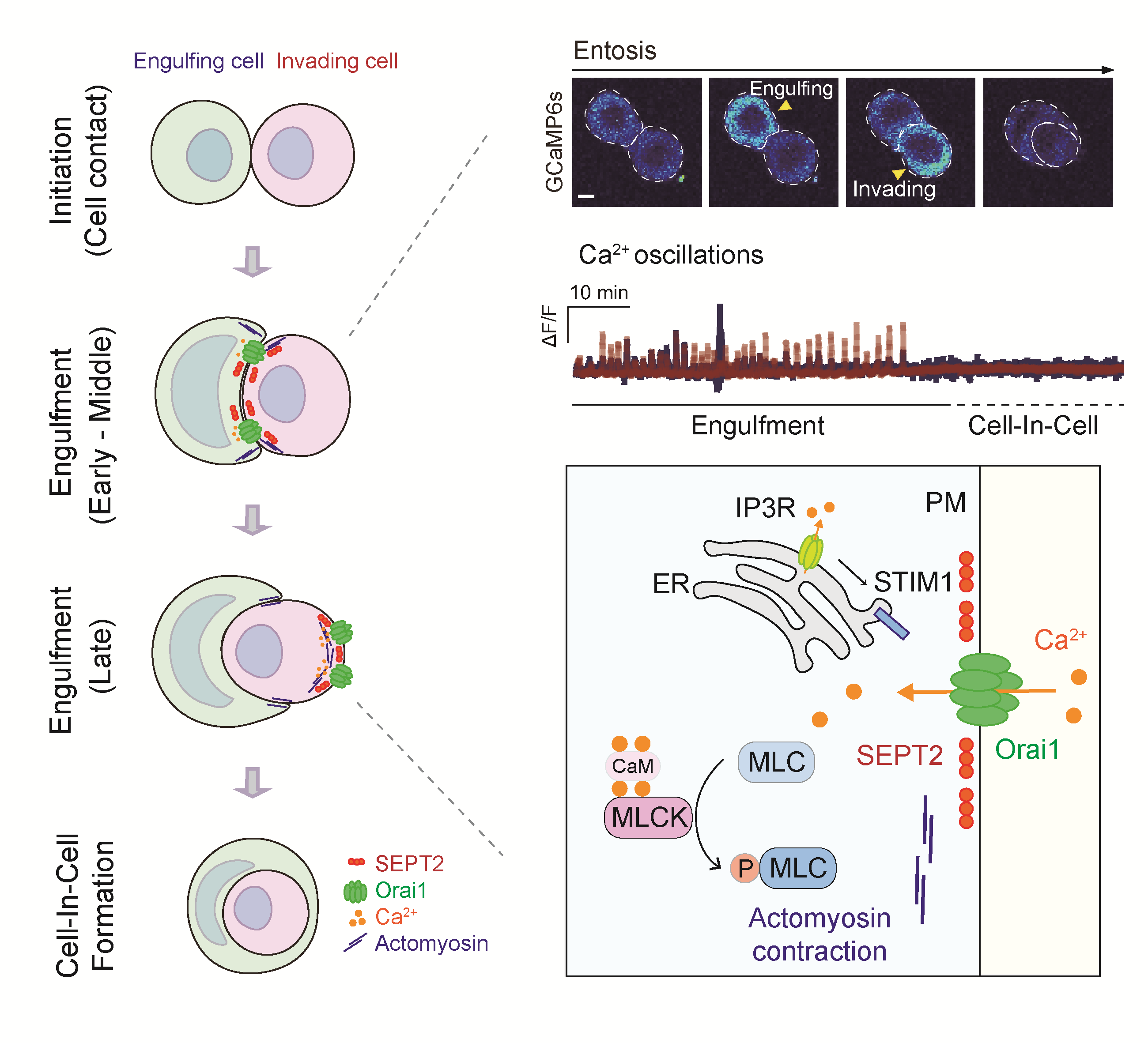
Figure. Entosis, a non-apoptotic cell death process, regulated by Orai1 Ca2+ channels.
During the early stages of cancer development, free-to-move cancerous cells (invasive and sporadic) cause unique signaling mechanisms within or between themselves. Calcium channel-dependent proteins are known to be crucial components involved in signaling processes among these malignant entities; however, no research has been conducted on their association with Entosis until now.
The research team, through their study, discovered that the Orai1 signaling mechanism – a calcium channel protein located in the cell membrane – is vital for inducing entosis in cancer cells. The team observed that Orai1 moves to specific areas of the cell membrane by septin (a cytoskeletal protein) and causes concentration changes in invasive and predatory cancer cells by exhibiting characteristic patterns at these locations.
These specific calcium signal transmission mechanisms phosphorylate myosin – an essential power protein responsible for cell movement- leading to rearrangement of the cytoskeleton or promoting cellular motility. This discovery enabled identification of the mechanism behind entosis induction and progression. Furthermore, controlling either Orai1 channels or signaling mechanisms can inhibit entosis thereby suggesting regulation of cancer incidence dependent on this process.
“This study identifies potential targets for treating entosis-associated tumors, showing that Orai1 is an entotic Ca2+ channel that provides essential Ca2+ signaling and sheds light on the molecular mechanism underlying entosis that involves SEPTIN filaments, Orai1, and MLCK,” noted Professor Park.
The findings of this research have been published in Advanced Science on May 17, 2023. This study has been supported through the Global Ph.D. Fellowship and Mid-Career Researcher Programs by the National Research Foundation of Korea (NRF) and the Ministry of Science and ICT (MSIT).
Journal Reference
Ah Reum Lee and Chan Young Park, “Orai1 is an Entotic Ca2+ Channel for Non-Apoptotic Cell Death, Entosis in Cancer Development,” Adv. Sci., (2023).