A groundbreaking advancement in diabetes treatment has been achieved by Professor Hyun-Wook Kang and his team from the Department of Biomedical Engineering at UNIST. They have developed a novel pancreatic islet macroencapsulation system designed for subcutaneous transplantation, offering a promising solution for Type 1 diabetes management.
Traditionally, pancreatic islets—clusters of cells responsible for insulin secretion—are transplanted into vascular-rich organs such as the liver or kidneys to treat severe insulin secretion disorders in Type 1 diabetic patients. This new graft allows for a minimally invasive procedure involving a simple incision for subcutaneous implantation, significantly reducing surgical complications and burden on the patient.
Historically, the liver and kidneys werec preferred transplant sites due to their abundant blood supply, which provides essential oxygen and nutrients, supports blood sugar detection, and facilitates insulin secretion. However, the low vascular density of subcutaneous tissue has presented challenges for islet survival and functionality.
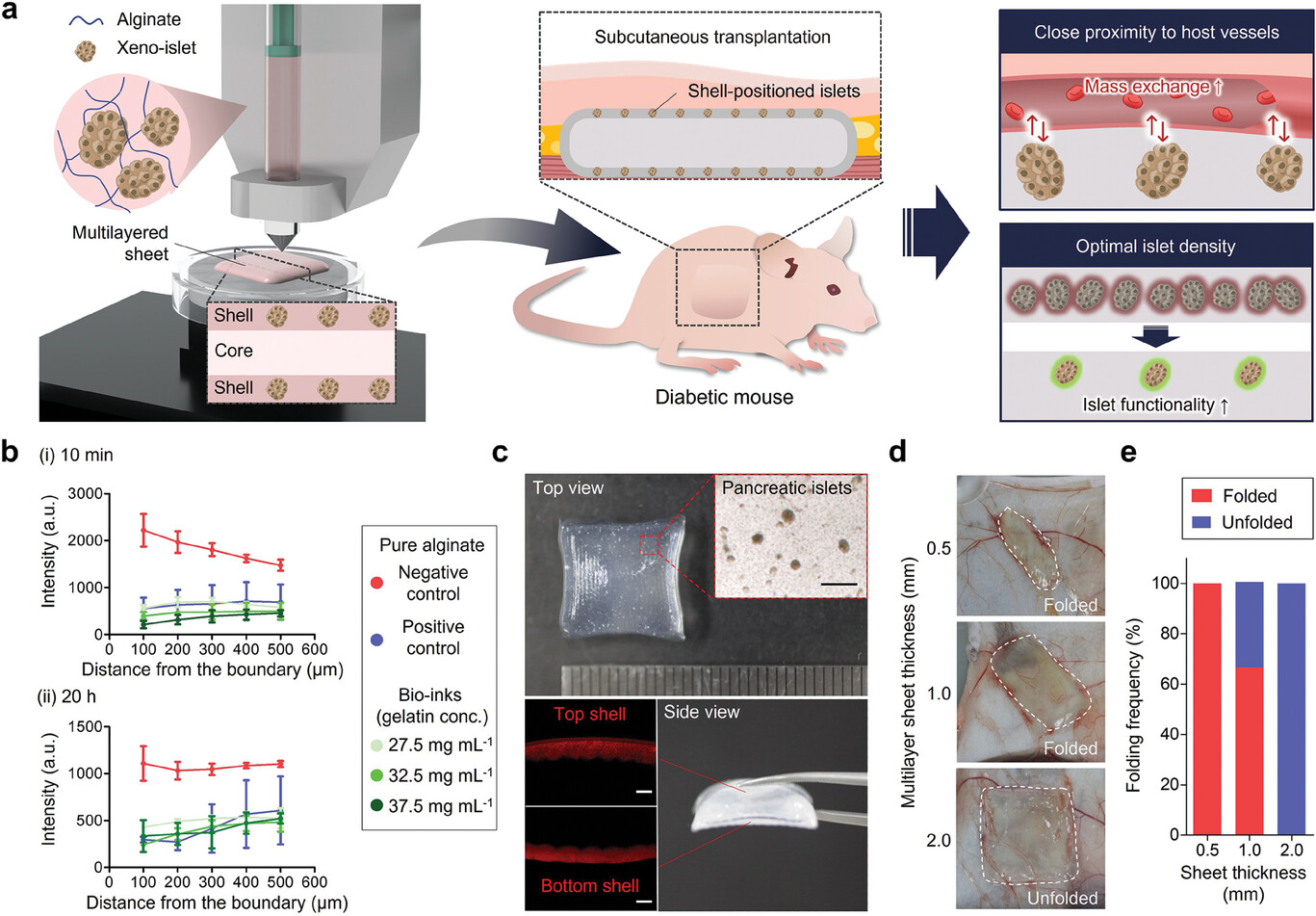
Figure 1. Schematic and physical properties of bioprinted islet macroencapsulation system.
To overcome these hurdles, the research team designed a multi-layered sheet-like structure that enhances islet efficacy in subcutaneous environments. The innovative graft positions pancreatic islets in top and bottom micro-shell layers, strategically minimizing the distance from surrounding blood vessels, thus optimizing oxygen and nutrient delivery. Each component of the implant, except for the islets, is biocompatible, further supporting the graft’s long-term viability.
The complex architecture of this implant was achieved through precision 3D bioprinting, allowing for customization to suit the transplantation needs of human patients. This advancement enhances the clinical applicability of the graft.
Additionally, the research opens new avenues for islet xenotransplantation, where animal pancreas is transplanted into humans. The design allows for the incorporation of antifibrotic drugs that inhibit immune responses, enabling localized drug delivery to improve graft acceptance.
In studies with diabetic mice, the newly developed pancreatic duct transplant demonstrated remarkable blood sugar regulation for over four months. The research team is now actively pursuing clinical trials and preparing for further animal studies.
Professor Kang stated, “This study represents a revolutionary approach to treating Type 1 diabetes. Our research could significantly enhance the quality of life for many individuals living with this challenging condition.”
Journal Reference
Seunggyu Jeon, Jun-Ho Heo, Noehyun Myung, et al., “High-Efficiency, Prevascularization-Free Macroencapsulation System for Subcutaneous Transplantation of Pancreatic Islets for Enhanced Diabetes Treatment,” Advanced Materials, (2024).












