A recent study, affiliated with UNIST has introduced a cheap and simple way to enhance the energy efficiency of metal-air batteries, using two different types of catalysts. This breakthrough has been led by Professor Guntae Kim and his research team in the School of Energy and Chemical Engineering at UNIST.
In this work, Professor Kim and his research team developed a new composite catalyst, namely ‘SSC-HG’ to enhance the performance of metal-air batteries. In this work, Professor Kim and his research team developed a new composite catalyst, namely ‘SSC-HG’ to enhance the performance of metal-air batteries. With the use of two different catalysts, the research team achieved a synergistic effect. Thus, they analyzed such phenomenon in the study and proposed future research directions.
A metal-air battery is equipped with an anode made up of pure metals—like lithium or zinc—and an air cathode that is connected to an inexhaustible source of air. Metal-air batteries produce electricity from the reaction of oxygen in the air with metals, such as Ca, Al, Fe, Cd, and Zn. Conversely, these batteries can also store electricity through the deoxidation process where oxygen is removed from oxidized metals. The currently existing metal-air batteries use rare and expensive metal catalysts for their air electrodes, such as platinum (Pt) and iridium oxide (IrO2). This has hindered its further commercialization into the marketplace.
In order to solve this problem, considerable efforts have been made to develop new catalysts through the application of coupled perovskite oxides/carbon materials. Besides, extensive research is being carried out to enhance the overall catalyst performance, using both materials.
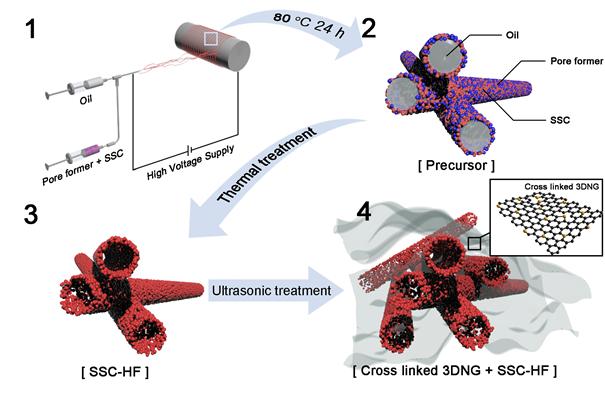
Manufacturing process of composite catalysts, namely ‘SSC-HG’.
In this work, Professor Kim’s team engineered composite catalysts, using ‘Perovskite‐type Sm0.5Sr0.5CoO3‐δ hollow nanofibers (SSC‐HF)’ and ‘Three-dimensional N‐doped graphene (3DNG)’. Both catalysts, when combined, create a synergetic effect, thus exhibiting high catalytic performance.
“When using a composite catalyst is used, it is common to only have one catalyst that is more beneficial for battery operating conditions,” says Seona Kim in the Combined MS/PhD program in the School of Energy and Chemical Eingeering at UNIST. “Yet, the newly-designed hybrid catalyst exhibits a remarkable enhancement in overall catalytic performance through the active interaction between two catalysts.”
Besides, the research team unveiled the cause of synergistic interactions between oxide and carbon via density functional theory (DFT) calculation. As a result, it was found that 3DNG with high electrical conductivity in the new composite catalyst is more active in oxygen reduction reaction (ORR) and oxygen generation reaction (OER) while transferring many electrons to SSC.
3DNG transfers electrons to oxygen molecules and creates ‘oxygen with high reaction energy’. This makes oxygen reduction reaction more likely to break oxygen molecules in cobalt (Co) on SSC surface connected with 3DNG. Conversely, when oxygen atoms are bonded, 3DNG, which has many electrons, strengthens the cobalt-oxygen covalent bond by giving electrons to the cobalt of SSC, thereby causing oxygen generation reaction better.

Pictured on the cover is Professor Kim’s synthesized, porous, perovskite‐type Sm0.5Sr0.5CoO3‐δ hollow nanofibers (SSC‐HF) are hybridized with cross‐linked, 3D, N‐doped graphene (3DNG).
“The synergistic effect shown in composite catalysts is the result of accelerating the electron transfer between the catalysts,” says Professor Kim. “The new composite catalyst will contribute to the metal air battery industry by ensuring performance as well as stability.”
This study has been also participated by Professor Jaephil Cho and Professor JunHee Lee in the School of Energy and Chemical Engineering at UNIST. Their work has been also featured on the cover page of the November 2018 issue of the prestigious journal, Small.
Journal Reference
Yunfei Bu, et. al., “A Tailored Bifunctional Electrocatalyst: Boosting Oxygen Reduction/Evolution Catalysis via Electron Transfer Between N‐Doped Graphene and Perovskite Oxides,” Small, (2018).