A research team, jointly led by Professor Tae-Eun Park and Professor Jinmyoung Joo in the Department of Biological Sciences at UNIST unveiled reliable BBB-penetrating aptamers that perform efficiently under human physiological conditions.
The blood-brain barrier (BBB) is a term used to describe the unique properties of the microvasculature of the central nervous system (CNS). It serves to protect the brain from the intrusion of external substances by allowing only substances essential for brain function. However, this system has been a major obstacle to drug treatment because it even controls drugs to treat brain diseases.
Currently, the ‘Trojan Horse’ technique is mainly used to effectively transport drugs to the brain. This method also enables receptor-mediated delivery of the fusion protein across the BBB so that the protein drug can enter the brain and exert the desired pharmacological effect. As such, short single-stranded DNA/RNA nucleotides forming a three-dimensional structure, called aptamers, have received increasing attention as BBB shuttles for efficient brain drug delivery owing to their practical advantages over Trojan horse antibodies or peptides, according to the research team.
“Aptamers are typically obtained by combinatorial chemical technology, termed Systemic Evolution of Ligands by EXponential Enrichment (SELEX), against purified targets, living cells, or animal models,” noted the research team. “However, identifying reliable BBB-penetrating aptamers that perform efficiently under human physiological conditions has been challenging because of the poor physiological relevance in the conventional SELEX process.”
To select aptamers that can recognize human BMEC and penetrate the BBB under physiological conditions, the research team developed a MPS-SELEX, which uses human BBB MPS as a complex target for aptamer selection.
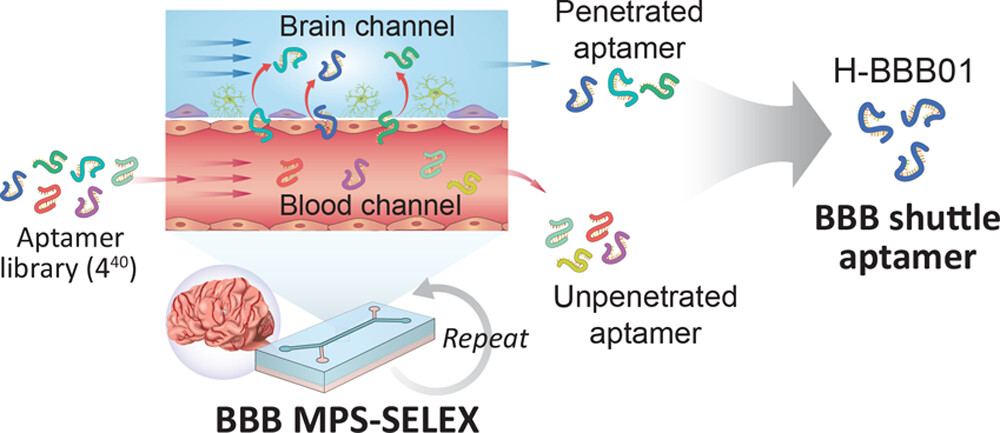
Figure 1. Schematic diagram of MPS-SELEX process.
After five-round of MPS-SELEX, the identified aptamer (hBS01) exhibited high capability to transport protein cargoes across the human BBB via clathrin-mediated endocytosis and enhanced uptake efficiency in BMECs and brain cells. The research team further validated the enhanced targeting specificity of hBS01 both in vitro and in vivo, thus confirming its powerful brain accumulation efficiency. According to the research team, these findings demonstrate that MPS-SELEX has potential in the discovery of aptamers with high target specificity that can be widely utilized to boost the development of drug delivery strategies.
“The current study exploited only BBB MPS to screen BBB penetrating aptamer; however, this approach can be further improved by integrating multiple organ models such as liver and kidney MPS in an MPS-SELEX process, which can filter out aptamers targeting other organs,” noted the research team. “Our proposed approach might therefore be useful in the discovery of aptamer-based drug delivery approaches for a variety of brain pathologies and can be applied more broadly in various organ MPSs.”
The findings of this study has been published in the April 2023 issue of ACS Nano, an internationally-renowned journal in the areas of nanoscience and nanotechnology.
Journal Reference
Jeong-Won Choi, Minwook Seo, Kyunghwan Kim, et al., “Aptamer Nanoconstructs Crossing Human Blood–Brain Barrier Discovered via Microphysiological System-Based SELEX Technology,” ACS Nano, (2023).