A research team, affiliated with UNIST has unveiled a novel surface processing technique that prolongs the lifespan of lithium-metal batteries (LMBs) while reducing explosion risk.
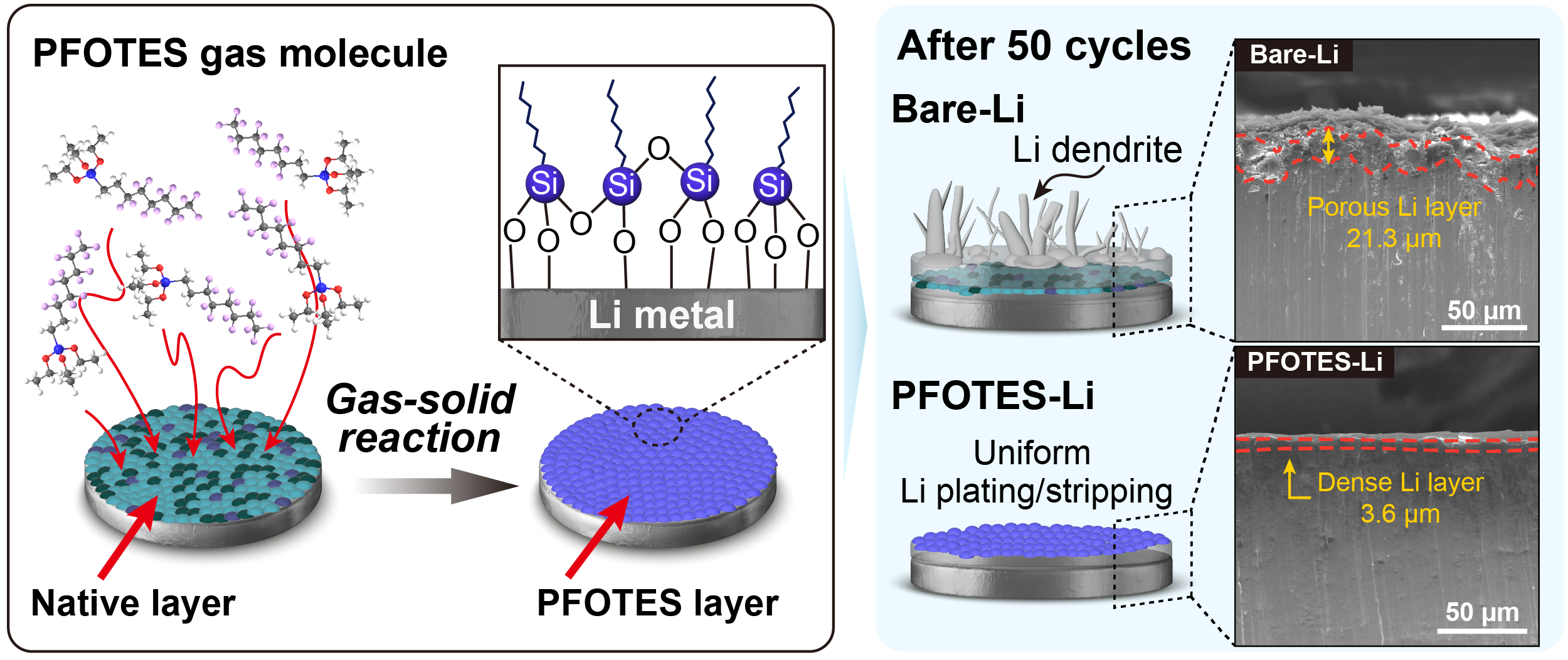
The team, led by Professor Hyeon Jeong Lee and Professor Seung Geol Lee from the Department of Materials Science and Engineering at UNIST, in collaboration with Professor Ji Hoon Lee’s team from Kyungpook National University, has devised a gas-phase reaction method that modifies the electrode surface of LMBs. This technology chemically reacts with the native passivation layer on lithium (Li) metal, removing it and simultaneously forming a stable, protective solid electrolyte interphase (SEI) layer.
LMBs, which utilize Li metal as the anode instead of graphite, are considered next-generation energy storage solutions due to their high energy density—potentially doubling the driving range of electric vehicles. However, the unstable native passivation layer on the lithium surface impedes uniform lithium deposition, shortens battery lifespan, and promotes dendrite growth during charging, which increases the risk of thermal runaway and explosions.
The research team innovated a gas-solid reaction process using fluoroalkyl silane, which enables the removal of the native oxide and carbonate-based passivation layer. This process results in the formation of a flexible, stable SEI composed of fluorinated carbon chains and a Si–O–Si network, providing an effective barrier that facilitates rapid lithium-ion transport.
Density Functional Theory (DFT) calculations revealed that the newly formed interface layer enhances the reversibility of lithium adsorption and desorption processes, promoting smoother lithium plating and stripping. In contrast, the native passivation layer was found to excessively adsorb lithium ions, hindering desorption and impairing the battery’s cycling stability.
Professor Seung Geol Lee explained, “Most previous studies on Li metal deposition focused solely on adsorption energies.” He added, “Our research comprehensively considers both deposition and desorption processes, providing a deeper understanding of the electrode’s reversibility.”
The engineered interface layer encourages uniform lithium-ion flow, suppressing dendrite growth and maintaining mechanical stability. This allows Li metal electrodes to operate reliably even in standard carbonate electrolytes without additional additives. Coin cell tests with high-capacity NMC811 cathodes (over 20 mg/cm²) demonstrated more than double the cycle life compared to conventional Li metal electrodes.
Professor Hyeon Jeong Lee emphasized, “This study not only removes the passivation layer, but also forms a protective, Li-ion-permeable coating, enabling long-term stable operation without reliance on electrolyte additives.” She further noted, “The process’s compatibility with relatively low temperatures (around 120°C) and its scalable gas-solid reaction approach open up broad possibilities for practical applications.”
This research was conducted by first authors Siwon Choi and Seongwook Chae from the Department of Materials Science and Engineering, supported by the National Research Foundation of Korea (NRF) and its New Generation Research Program. The findings were published in the renowned journal ACS Nano on April 29, 2024.
Journal Reference
Siwon Choi, Seongwook Chae, Taemin Kim, et al., “Strategic Surface Engineering of Lithium Metal Anodes: Simultaneous Native Layer Elimination and Protective Layer Formation via Gas–Solid Reaction,” ACS Nano, (2025).