A groundbreaking anticancer treatment technology that selectively targets cancer cell lysosomes and overcomes drug resistance has been developed by Professor Ja-Hyoung Ryu and his research team in the Department of Chemistry at UNIST. This pioneering research promises a new paradigm for chemical anticancer drugs in the future.
Lysosomes are crucial organelles responsible for breaking down and recycling cellular components. Targeting lysosomes with anticancer drugs has emerged as a promising approach to combat drug resistance in cancer cells. However, until now, extensive research in this area has been lacking.
The research team designed a novel material capable of self-assembling into micelle structures following specific rules. Micelles are spherical structures with an oil-friendly interior surrounded by a water-friendly exterior. These micelle structures exhibit excellent stability within the in vivo environment while remaining non-toxic to surrounding cells.
Significantly, these micelles incorporate ‘RGD peptides,’ known for their selective targeting ability towards receptors overexpressed on cancer cell membranes. Given that cancer cell lysosomes often exhibit high levels of the enzyme ‘Cathepsin B,’ responsible for protein degradation, the micelles specifically target these lysosomes. Once inside the lysosome, they interact with Cathepsin B.
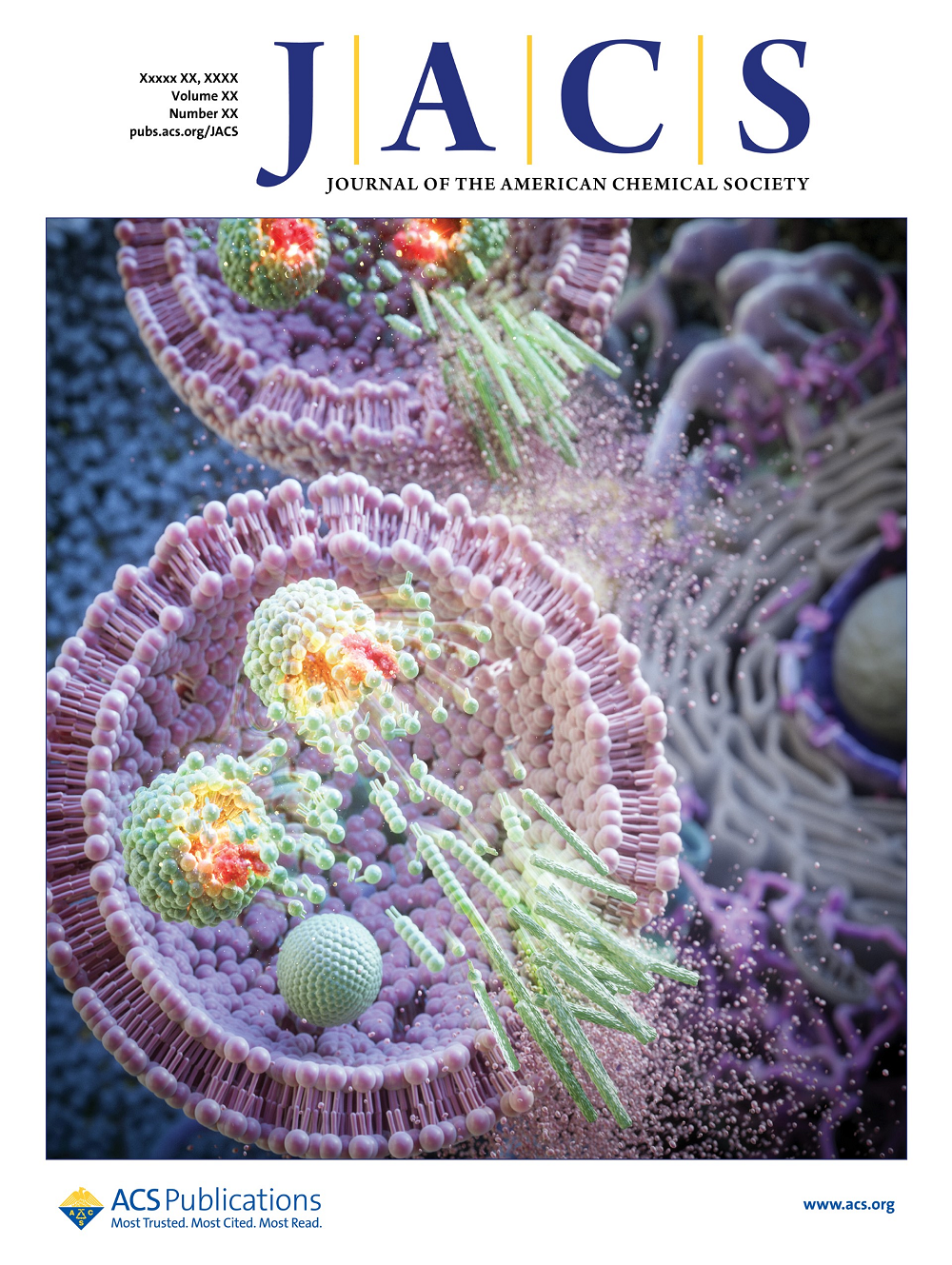
The study findings have been featured as the cover page of the online version of JACS on July 17, 2023.
As a result, specific regions of the peptide within the micelle structure are cleaved by Cathepsin B enzymes. The resulting cut molecules then reassemble into long fiber-like structures through self-assembly processes, causing damage to the lysosomal membrane. Ultimately, this leads to dysfunctional lysosomes and subsequent apoptotic death of cancer cells.
Lead authors, Research Professor Batakrishna Jana (Department of Chemistry, UNIST) and Seongeon Jin (Korea Institute of Science and Technology, KIST), stated, “We have demonstrated cancer cell death by inducing lysosomal reassembly based on the overexpression of Cathepsin B in cancer cells.”
This developed substance stands out for its ability to overcome drug resistance—a significant drawback of conventional chemical anticancer drugs—while improving target capabilities. Conventional chemotherapy often faces resistance due to continuous drug administration, but this new approach selectively disrupts cancer cell lysosomes, circumventing such resistance.
Professor Yoo remarked, “Targeting cancer cell lysosomes allows for effective anticancer treatments without encountering drug-resistant challenges,” adding that this research opens up a new vision for chemical-based anticancer treatments in the future.
The collaborative research involved Professor Sang Kyu Kwak from the Department of Chemical and Biological Engineering at Korea University, and received support from the Mid-Career Researcher Project and the Bio·Medical Technology Development Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (MSIT). The study findings have been published ahead of their official publication in the online version of the Journal of the American Chemical Society (JACS) on July 17, 2023.
Journal Reference
Batakrishna Jana, Seongeon Jin, Eun Min Go, et al., ‘Intra-Lysosomal Peptide Assembly for the High Selectivity Index against Cancer,’ J. Am. Chem. Soc., (2023).