Recent advancements have significantly reduced the risk of lithium (Li) battery explosions, paving the way for safer and more reliable energy storage solutions. Researchers have demonstrated that battery safety and longevity can be greatly enhanced by mitigating dendritic Li growth using metal foils with precisely arranged copper atoms.
A research team, led by Professor Hyun-Wook Lee from the School of Energy and Chemical Engineering at UNIST utilized single-crystal Cu(111) foil, produced through innovative contactless heat treatment, in anode-free Li batteries. This advanced foil facilitates the uniform distribution of Li across the battery surface, effectively preventing dendrite formation. As a result, the implementation of anode-free Li batteries now comes with minimal risk of explosion.
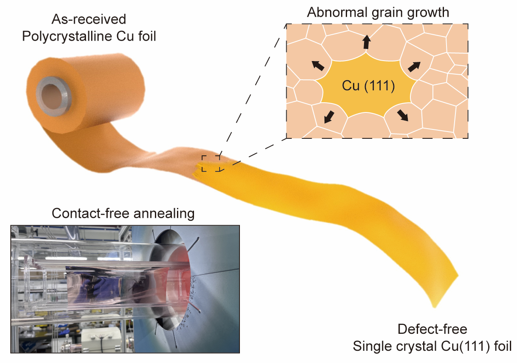
Figure 1. Illustration of the preparation of a single crystal Cu(111) foil. Cu(111) foil is prepared via abnormal growth of grains with (111) orientation.
The research team successfully addressed the issue of dendrite formation—irregular, branch-like structures that develop unevenly during Li stacking—by promoting horizontal rather than vertical lithium growth on the single-crystal Cu(111) foil. Dendrites are a primary cause of increased explosion risk due to their potential to create electrical short circuits.
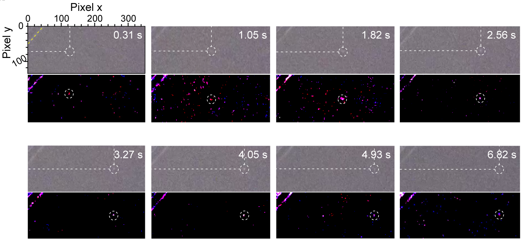
Figure 2. Optical images of the surface migration of a Li adatom on a Cu(111) upon Li plating for roughly 10s and its trajectory .
Historically, dendrite formation during the charging process has posed a significant barrier to the commercialization of Li metal batteries. Collaborating with distinguished experts, including Professor Rodney S. Ruoff, Director of the IBS Center for Multidimensional Carbon Materials (CMCM), Professor Sunghwan Jin from Kangwon National University, and Professor Dong-Hwa Seo from KAIST, the team confirmed that enhancing the uniformity of lithium growth resulted in a stable tetrahedral crystal structure, thereby improving both efficiency and safety.
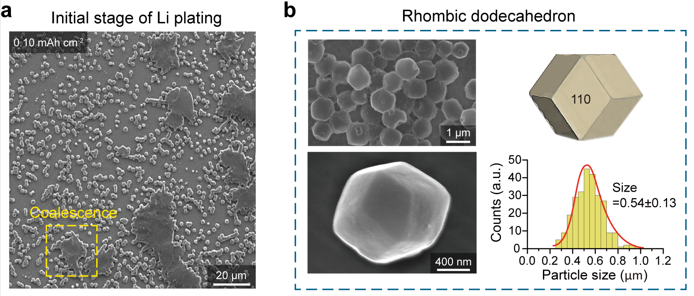 Figure 3. The formation of Li rhombic dodecahedra followed by horizontal Li growth. (a) SEM image of electrodeposited Li on a Cu(111) foil and polycrystalline Cu foils with an areal capacity of 0.10 mA h cm−2, showing the coalescence of Li particles. (b) SEM images of rhombic dodecahedral Li particles on a Cu(111) foil and their size distributions (mean = 54 ± 13 nm).
Figure 3. The formation of Li rhombic dodecahedra followed by horizontal Li growth. (a) SEM image of electrodeposited Li on a Cu(111) foil and polycrystalline Cu foils with an areal capacity of 0.10 mA h cm−2, showing the coalescence of Li particles. (b) SEM images of rhombic dodecahedral Li particles on a Cu(111) foil and their size distributions (mean = 54 ± 13 nm).
In their comparative studies of Li growth patterns on various Cu foils, the team discovered that irregular arrangements of high Miller index crystal planes contribute to dendrite formation. This finding delivers critical insights for the development of next-generation metal substrates that could revolutionize battery design.
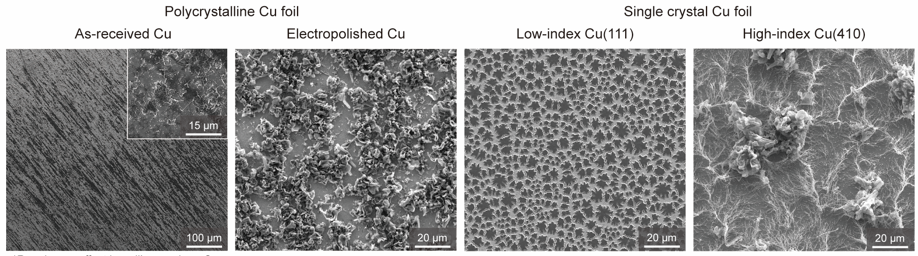 Figure 4. Li morphologies on four different Cu foils with areal capacity of 0.5 mA h cm−2: as-received Cu, electropolished polycrystalline Cu, low-index single crystal Cu(111), and high-index single crystal Cu(410).
Figure 4. Li morphologies on four different Cu foils with areal capacity of 0.5 mA h cm−2: as-received Cu, electropolished polycrystalline Cu, low-index single crystal Cu(111), and high-index single crystal Cu(410).
“Previous Li battery research has focused primarily on charging,” noted Professor Lee. “Our findings signify a paradigm shift in Li growth from vertical to horizontal, offering a promising pathway toward the realization of commercial anode-free Li batteries.”
This groundbreaking study was conducted with support from UNIST, the Ministry of Science and ICT (MSIT), the National Research Council of Science and Technology (NST), the Institute for Basic Science (IBS), and through the International Joint Research on Secondary Batteries by the National Research Foundation of Korea (NRF). Their findings were selected as the inner front cover of Energy & Environmental Science on September 21, 2024.
Journal Reference
Min-Ho Kim, Dong Yeon Kim, Yunqing Li, et al., “Horizontal lithium growth driven by surface dynamics on single crystal Cu(111) foil,” Energy Environ. Sci., (2024).